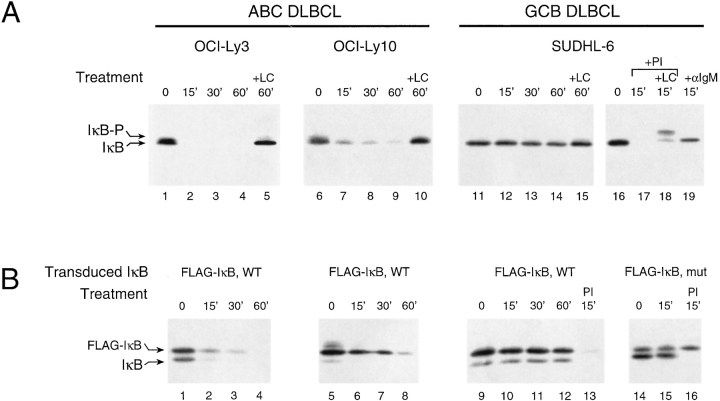
Figure 3.
(A) Rapid, constitutive, and proteasome-dependent degradation of IκBα in ABC DLBCL cell lines. Western blot analysis of IκBα in extracts of CHX-treated cells (5 × 105 cells per lane, 20 μg/ml CHX). SUDHL-6 showed stable IκBα unless cells were activated for the times indicated by PMA (Sigma-Aldrich; 40 ng/ml plus ionomycin) (Calbiochem; 2 μM) (PI) or anti-IgM (50 μg/ml). The more slowly migrating form of IκBα (IκB-P) seen in cells treated with clastolactacystin β−lactone (LC; Calbiochem, 80 μM), the active metabolite of lactacystin, most likely represents phosphorylated IκB. (B) Ectopically expressed wild-type IκBα was degraded similarly to endogenous IκBα in ABC and GCB DLBCL cell lines. Western blot analysis of IκBα in extracts of CHX-treated cells stably transduced with retroviruses expressing FLAG epitope-tagged wild-type IκBα or super-repressor IκBα (S32G/S36A) and treated with CHX with and without PI for the times indicated. In SUDHL-6, the super-repressor form of FLAG-tagged IκBα did not affect the degradation of endogenous IκBα induced by PI but was itself resistant to degradation.