Figure 3.
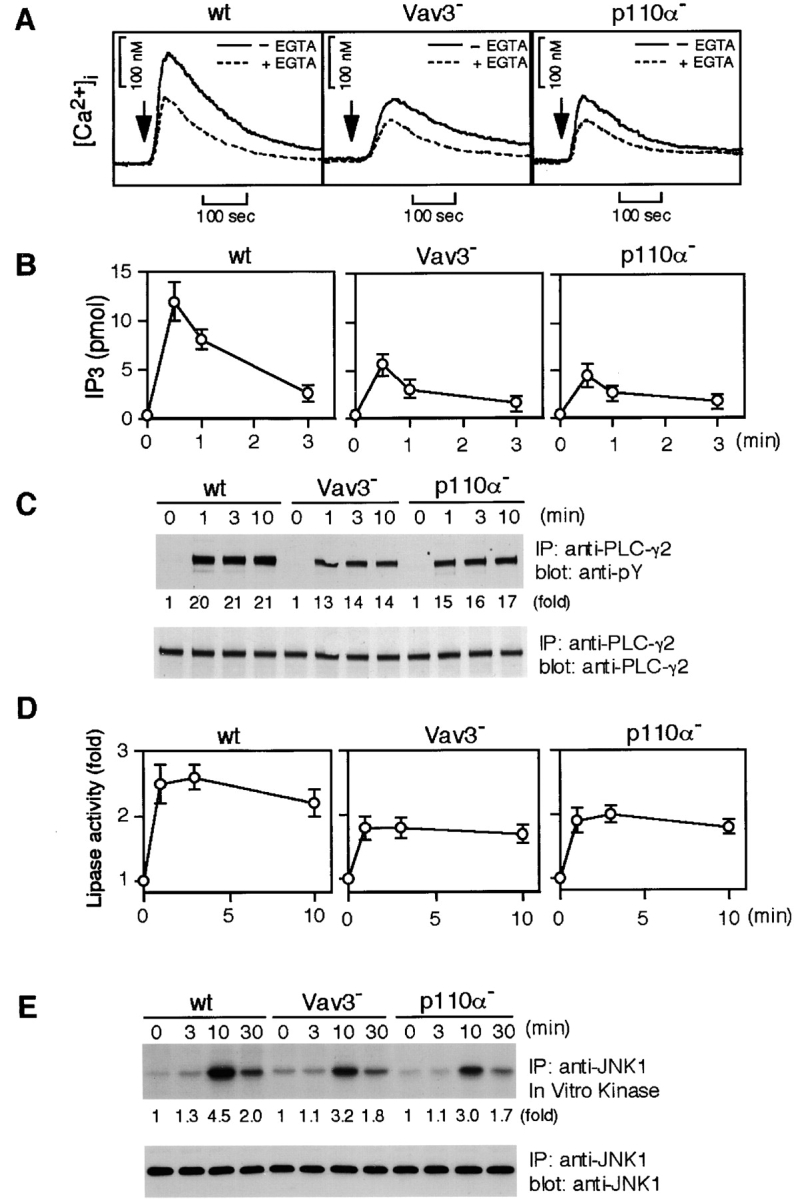
Characterization of Vav3- and PI3K p110α-deficient DT40 B cells. Cells were stimulated with M4 (4 μg/ml) for indicated time periods and then subjected to the following analyses. (A) Calcium mobilization. Intracellular free calcium levels in Fura-2-loaded cells were monitored by a fluorescence spectrophotometer. Calcium release from intracellular calcium stores was measured in the presence of 1 mM EGTA (dotted line). wt, wild-type. (B) IP3 generation. Soluble IP3 was extracted from 2 × 106 cells and subjected to a Biotrak competitive binding assay system. The results (2 × 106 cell equivalents per point) were shown by mean ± standard error of three independent experiments. (C) Tyrosine phosphorylation of PLC-γ2. Immunoprecipitation (IP) was performed with anti-PLC-γ2 Abs. The blots (5 × 106 cell equivalents per lane) were stained with 4G10 mAb (top). The blots were stripped and reprobed with anti-PLC-γ2 Abs (bottom). (D) BCR-induced PLC-γ2 activation. PLC-γ2 was immunoprecipitated from lysates (2 × 107 cell equivalents per point), and in vitro phospholipase activity was measured as described in Materials and Methods. The results were shown by mean ± standard error of three independent experiments. (E) BCR-induced JNK1 activation. Lysates from 5 × 106 cells were immunoprecipitated with anti-JNK1 mAb, and the resulting immunoprecipitates were divided. Half of them was used for Western blot analysis using anti-JNK1 mAb (bottom). The remaining half was used for in vitro kinase assay using GST-c-Jun as an exogenous substrate. The kinase reaction products were resolved on 12.5% SDS-PAGE, and their phosphorylation was quantified by autoradiography (top).
