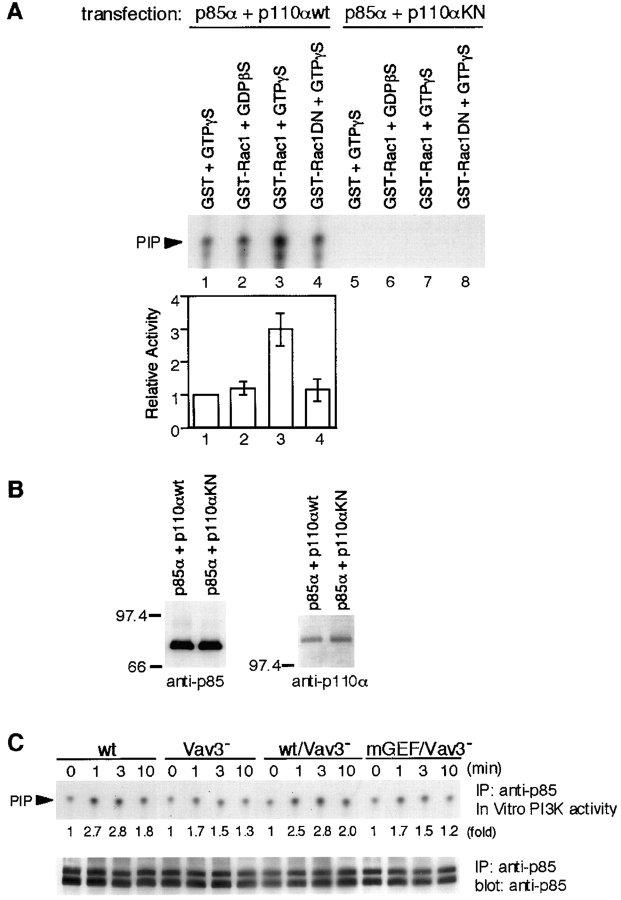
Figure 7.
PI3K enzymatic activity is upregulated by Rac1 in a GTP-dependent manner. (A and B) Effect of Rac1 on PI3K activity in vitro. (A) p85 was immunoprecipitated from lysates of 293T cells transfected with plasmids encoding either wild-type (wt; lane 1–4) or kinase-negative (KN; lane 5–8) p110α, together with p85α. Immunoprecipitates were mixed with GST/GTPγS (lane 1 and 5), GST-Rac1/GDPβS (lane 2 and 6), GST-Rac1/GTPγS (lane 3 and 7), or GST-Rac1N17/GTPγS (lane 4 and 8), and PI3K activity was assayed as described in Materials and Methods. In the top panel, one of representative results is shown. The bottom panel shows quantitative analysis of PI3K activity. The intensity of the PIP spots revealed in autoradiogram was measured by scanning densitometry. The activity in the presence of GST/GTPγS was used to normalize individual activities in each experiment. The results were shown by mean ± standard error of three independent experiments. (B) Aliquots of immunoprecipitates with anti-p85 Abs were subjected to Western blot analysis using anti-p85 Abs (left) or anti-p110α Abs (right). (C) BCR-induced PI3K activity. Lysates from 5 × 106 cells were immunoprecipitated with anti-p85 Abs, and the immunoprecipitates were subjected to PI3K assay. One of the representative results is shown in the top panel. Aliquots of immunoprecipitates were used for Western blot analysis with anti-p85 Abs (bottom). The doublet bands shown in the immunoblot are presumably due to p85α and p85β isoforms, as we used anti-pan-p85 Abs. IP, immunoprecipitation.