Figure 6.
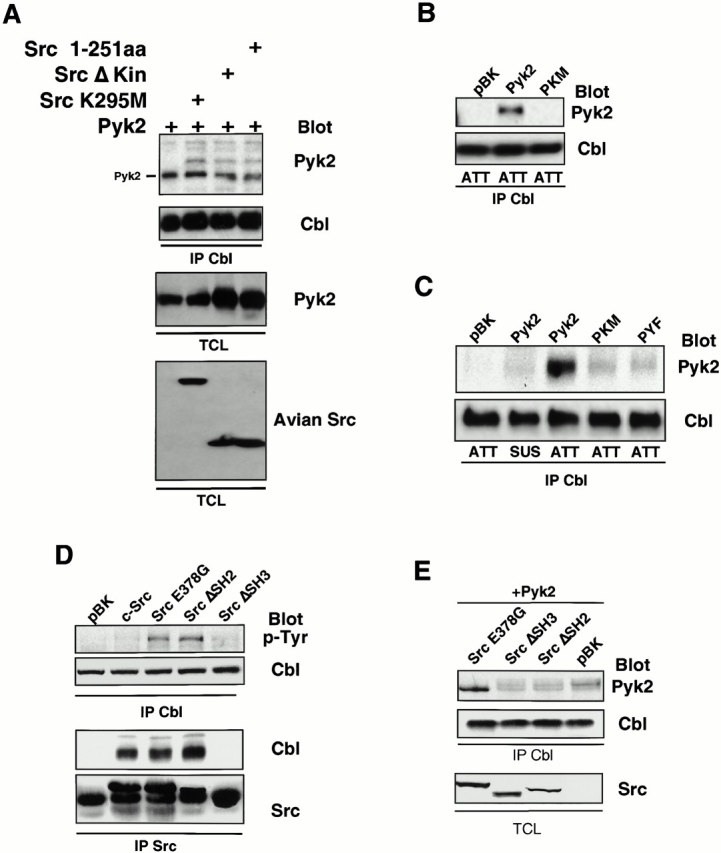
Src acts as an adaptor molecule mediating interactions between Cbl and Pyk2. (A) Association of Cbl with Pyk2 in presence of Src kinase mutants was analyzed by probing the blot of Cbl immunoprecipitates with Pyk2 antibodies (top). The blot was stripped and reprobed with Cbl antibodies. Total cell lysates (TCL) were Western blotted with Pyk2 and avian Src antibodies to check expression of transfected proteins. (B) 293-VnR cells were transfected with Pyk2, PKM, or empty vector (pBK). Cbl was immunoprecipitated from lysates of replated cells and analyzed for the presence of phosphotyrosine (top). (C) 293-VnR cells were transfected with Pyk2 mutants. The attachment-induced association of the mutant Pyk2 proteins with Cbl (top) was analyzed by blotting Cbl IPs with anti–Pyk2 antibody. (D) Cbl IPs from lysates of 293-VnR cells transfected with 10 μg of the indicated Src expression vector were blotted with an antiphosphotyrosine antibody to demonstrate the effects of these Src mutants of Cbl phosphorylation. Src was immunoprecipitated from the same lysates and the immune complexes were blotted for the presence of Cbl. (E) 293-VnR cells were transfected with a combination of 3 μg Pyk2 and 3 μg of the indicated Src expression vector. Cbl IPs were analyzed for the presence of Pyk2 (top). (Bottom) Expression of the Src mutants in total cell lysates (TCL).
