Figure 8.
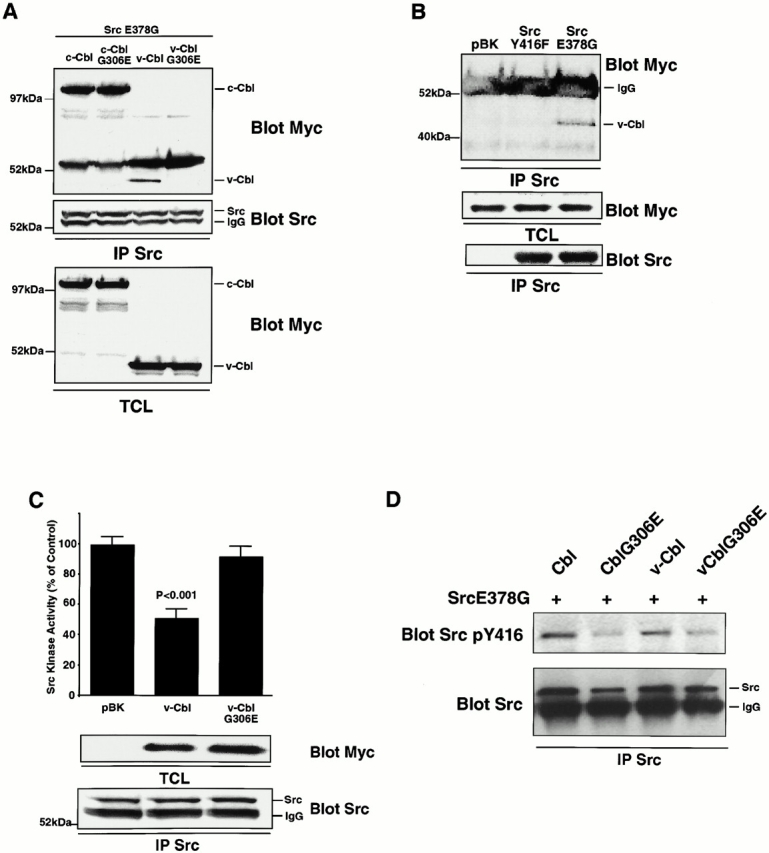
The association of the Cbl PTB domain with Src phosphotyrosine residue 416 results in an inhibition of Src kinase activity. (A) 293-VnR cells were transiently cotransfected with 5 μg Src E378G and 5 μg of normal or G306E-mutated forms of Cbl and v-Cbl. After immunoprecipitating Src, immune complexes were blotted for associated Cbl protein (top). The efficiencies of Src immunoprecipitation (middle) and Cbl expression (bottom) were determined. (B) 293-VnR v-Cbl cells were transiently transfected with active avian Src (SrcE378G) or avian Src Y416F. Avian Src was immunoprecipitated and blotted for the presence of myc-tagged v-Cbl. v-Cbl expression (middle) and efficiency of Src immunoprecipitation (bottom) were analyzed. (C) The kinase activity of immunoprecipitated Src from cells transfected with a combination of 5 μg SrcE378G and 5 μg of either v-Cbl or v-CblG306E was measured and expressed as a percentage of the control kinase activity levels (pBK-transfected cells). v-Cbl expression (middle) and efficiency of Src immunoprecipitation (bottom) were analyzed. (D) 293-Vnr cells were transiently cotransfected with 5 μg Src E378G and 5 μg normal or G306E mutated form of Cbl and v-Cbl. Src was immunoprecipitated and immune complex was immunoblotted with SrcY416 (top). The blot was reprobed with Src (bottom).
