Figure 4.
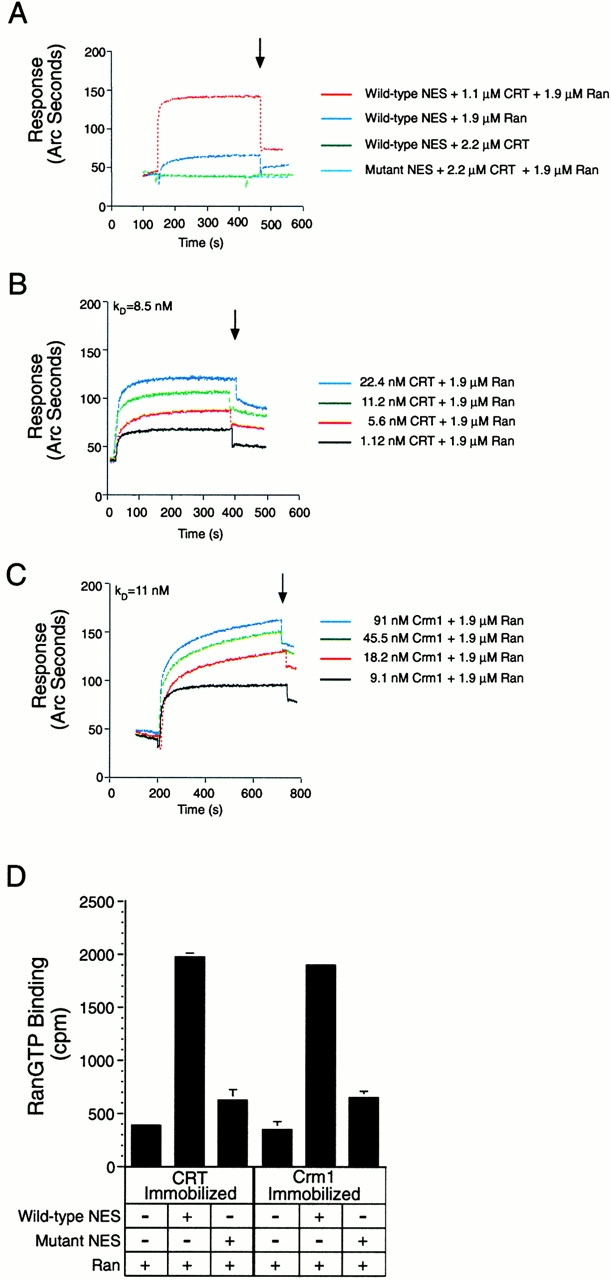
CRT forms a trimeric complex with the leucine-rich NES and RanGTP. A synthetic peptide containing the NES of PKI (WT or NES mutant) was immobilized on the surface of the biosensor cuvette through a biotin–STV linkage. The binding response of recombinant proteins to immobilized peptide was measured as the increase in refractive index as a function of time. (A) CRT binding to the WT NES requires the presence of RanGTP. GST–CRT (50 μg/ml) and Ran preloaded with GMP-PNP (50 μg/ml) were added alone, or in combination, to cuvettes containing immobilized WT NES or mutant NES (L41,44A). The arrow indicates the time at which buffer (PBS) was infused. Complex formation is observed in the presence of a WT NES, CRT, and RanGTP (red tracing). CRT and Ran do not form a complex on a mutant NES (light blue tracing), and only background levels of binding are observed if CRT or Ran is omitted from the assay (dark blue and green tracings, respectively). Note that the light blue and green tracings overlap at ∼40 arc s, which represents background binding. (B) Measurement of the affinity of CRT for NES in the presence of RanGTP. The association rate of CRT for NES in the presence of Ran was measured over a concentration range of CRT (1.12–22.4 nM). The dissociation rate was measured in a similar manner after infusion of buffer (beginning at 400 s), and the FASTfit program was used to calculate the affinity of CRT for NES in the presence of Ran (K D = 8.5 nM). (C) Measurement of the affinity of Crm1 for NES in the presence of RanGTP. The methods described above were used to measure the association and dissociation rates of the trimeric complex, and the FASTfit program was used to calculate the affinity (K D = 11 nM). (D) RanGTP binding to CRT and Crm1 is stimulated by the NES. His-tagged CRT and Crm1 were adsorbed to microtiter wells, and binding of Ran was measured in the absence and presence of the WT and mutant NES peptides. Since the recombinant Ran was preloaded with GTP radiolabeled on the terminal phosphate, the counts measured in the bound fraction specifically reflect the triphosphate form of Ran. Including WT NES in the binding reaction stabilizes the interaction of RanGTP with both CRT and Crm1.
