Figure 3.
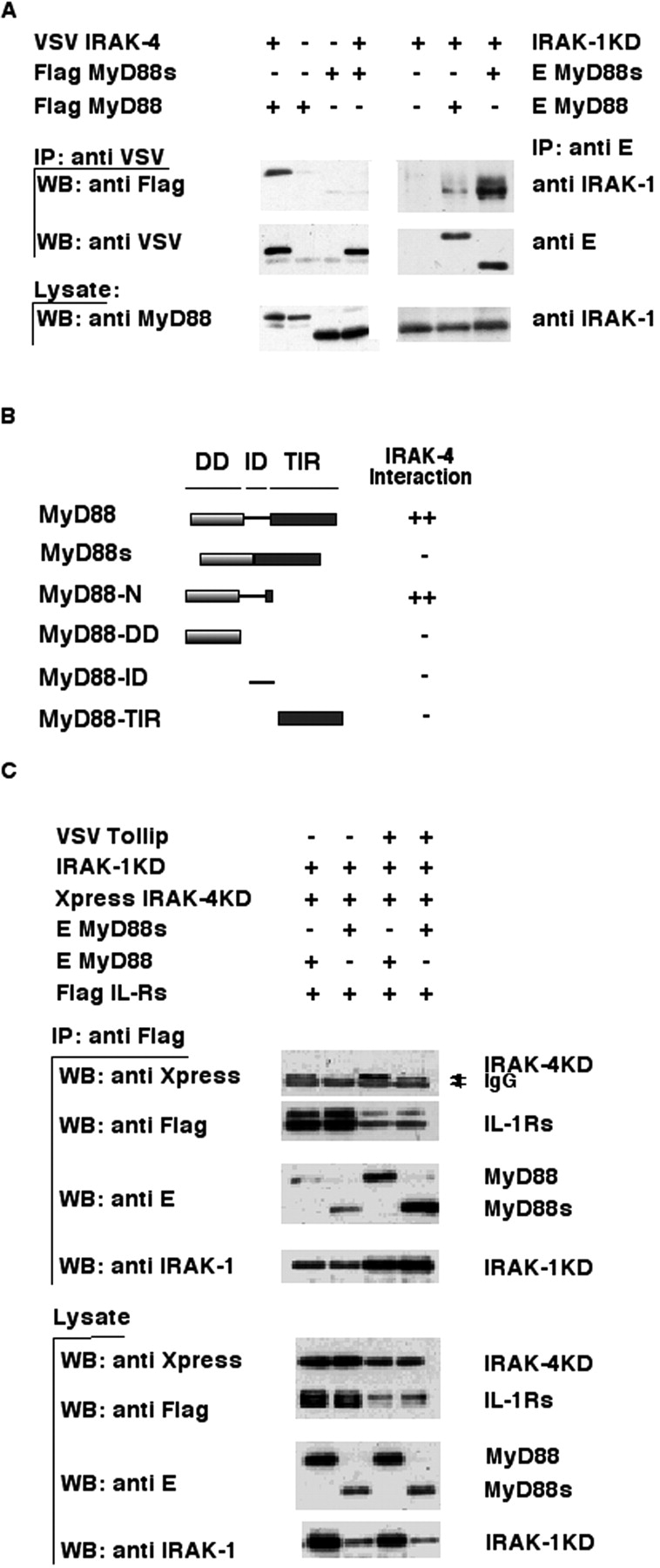
MyD88s does not bind IRAK-4 and blocks its recruitment to the IL-1Rs. (A) The left side of the top panel shows that IRAK-4 was coimmunoprecipitated using an anti-VSV antibody from lysates of 293T cells transfected with the indicated expression plasmids, and immuno-blotted with an anti-Flag antibody. The middle and bottom panels show Western blotting of immunoprecipitates and cell lysates, respectively, using anti-VSV or anti-MyD88 antibodies. The ride side of the top panel shows that MyD88 was coimmunoprecipitated using an anti-E antibody from lysates of 293T cells cotransfected with the indicated expression plasmids, and immunoblotted with an anti–IRAK-1 antibody. The middle and bottom panels show Western blotting of immunoprecipitates or cell lysates using anti-E (middle) or anti–IRAK-1 (bottom) antibodies. IP, immunoprecipitate; WB, Western blot. (B) HF7c yeast was cotransformed with expression vectors encoding the pGBT9 GAL4 DNA-binding domain fused to various MyD88 deletion mutants (schematically illustrated) and pGAD10 IRAK-4 expressing the GAL4 transcription activation domain fused to full-length IRAK-4. Interaction of the proteins was assessed by β galactosidase expression filter assays. ++ indicates strong color development within 60 min of the assay and − indicates no development of color within 24 h. All pGBT9 fusion proteins were checked for autoactivation and found to be negative. (C) Flag-tagged IL-1Rs were coimmunoprecipitated with anti-Flag antibodies from lysates of 293T cells cotransfected with the indicated expression plasmids and immunoblotted with an anti-Xpress antibody to detect IRAK-4KD association. The same blot was reprobed with anti-Flag, anti-E, and anti–IRAK-1 antibodies to monitor IL-1Rs, MyD88, and IRAK-1KD levels, respectively. Bottom panels show Western blotting of cell lysates using the indicated antibodies.
