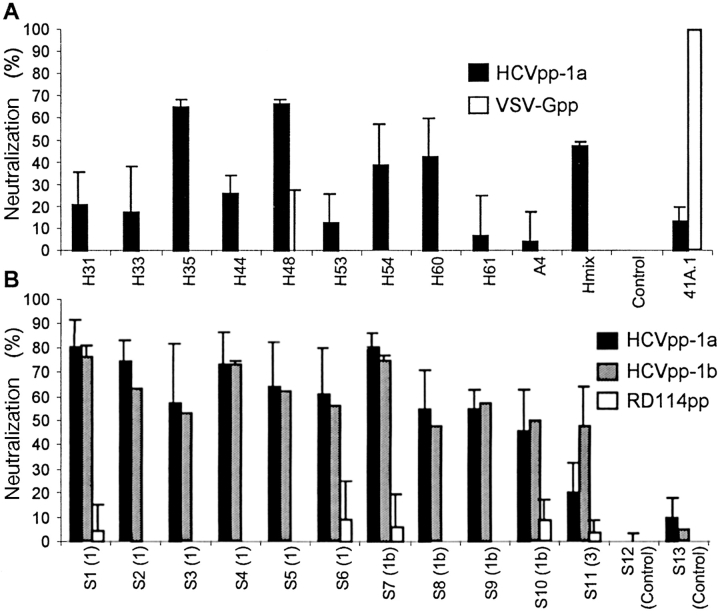
Figure 5.
Neutralization of HCV pseudo-particles. (A) Results of neutralization of HCVpp-1a generated with HCV-1a E1E2 and with MLV core proteins preincubated before infection of Huh-7 cells with 20 µg/ml saturating concentrations of monoclonal antibodies against E1 (A4) or E2 (H31, H33, H35, H44, H48, H53, H54, H60, and H61) glycoproteins of genotype 1a. Hmix: neutralization assays with pooled antibodies. Negative control experiments were performed using no antibodies (Control) or using pseudo-particles generated with VSV-G (VSV-Gpp). Neutralization of the infectivity of these control pseudo-particles was achieved by using the VSV-G neutralizing 41A.1 monoclonal antibody. Results are expressed as the percentages of inhibition of the average infectious titers ± SD relative to incubation in the absence of antibodies. (B) Results of neutralization of HCVpp with HCV patient sera. HCVpp of genotypes 1a or 1b were preincubated for 30 min at room temperature with sera from chronically HCV-infected patients diluted 1:50 before infection of Huh-7 target cells. The genotype of HCV diagnosed in these patients is indicated in brackets. Results are expressed as percentages of inhibition of the average infectious titers ± SD relative to incubation with control sera from healthy individuals. Control experiments were performed using pseudo-particles generated with RD114 glycoproteins (RD114pp), rather than with VSV-G, which exhibits sensitivity to human complement (15). Efficient neutralization of the control pseudo-particles (not depicted) was demonstrated with a hyper-immune goat serum raised against the RD114 SU glycoprotein.