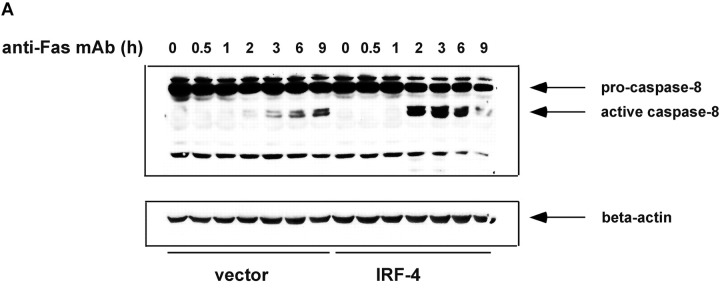
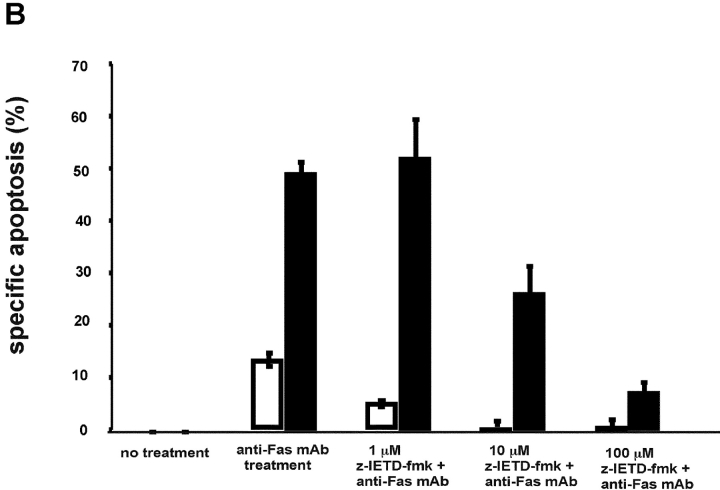
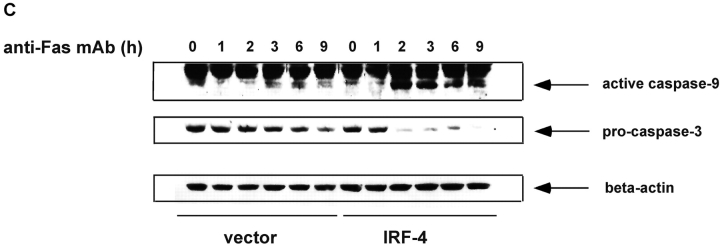
Figure 3.
Stable expression of IRF-4 leads to increased activation of caspase-8 and of downstream caspases upon engagement of the Fas receptor. (A) Cells from control and IRF-4 transfectants were cultured with the anti-Fas mAb CH-11 at 100 ng/ml and harvested after the indicated periods of incubation. Total cell lysates were separated by 7% SDS–polyacrylamide gel electrophoresis, and caspase-8 processing was detected by immunoblotting with an anti–human caspase-8 Ab (top). Vector refers to the control Jurkat transfectants, whereas IRF-4 refers to the IRF-4 stable Jurkat transfectants. The procaspase-8 form at 55 kD and the active caspase-8 at 40 kD are indicated by arrows. Blots were stripped and reprobed with a β-actin Ab as a loading control (bottom). (B) Open bars: control Jurkat transfectants; solid bar: IRF-4 Jurkat transfectants. Vector control and IRF-4 transfectants were cultured with or without z-IETD-fmk, a caspase-8 inhibitor, for 1 h at 37°C at 1-, 10-, or 100-μM concentration followed by the addition of 100 ng/ml anti-Fas mAb for 6 h. After this incubation, cells were harvested and analyzed by flow cytometry for apoptosis as indicated in Fig. 1 B. Addition of z-IETD fmk alone had no effect on the viability of the cells (unpublished data). (C) Cells were incubated with 100 ng/ml anti-Fas mAb CH-11 and harvested after the indicated periods of incubation. Total cell lysates were separated by SDS–polyacrylamide gel electrophoresis, and caspase-9 and -3 processing was detected by immunoblotting with antibodies against either active human caspase-9 (top) or procaspase-3 (middle). The active caspase-9 form at 37 kD and the procaspase-3 form are indicated by arrows. Blots were stripped and reprobed with a β-actin Ab as a loading control (bottom).