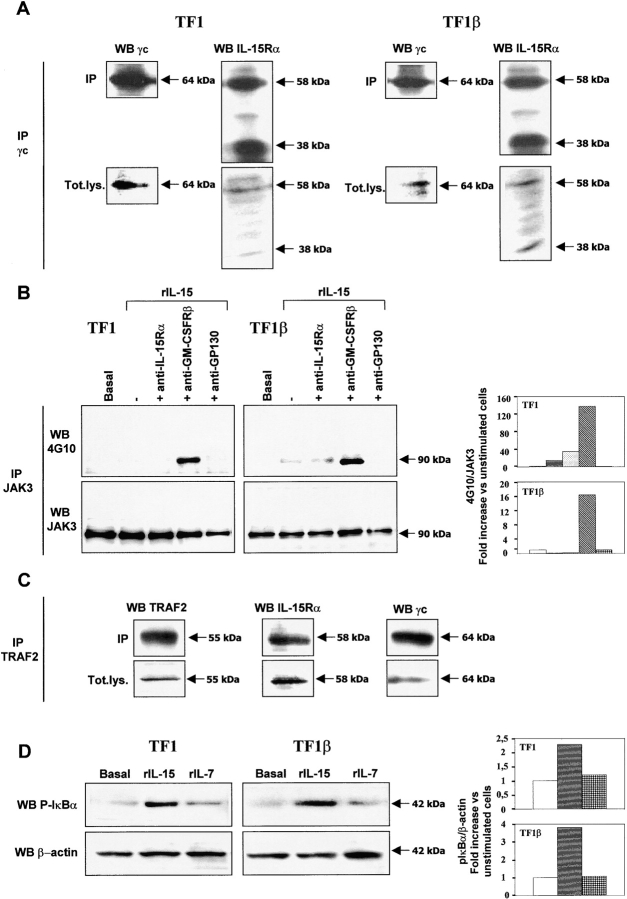
Figure 5.
IL-15Rα/γc interaction in TF1 and TF1β cells. (A) Coimmunoprecipitation. TF1 and TF1β cells were analyzed for IL-15Rα/γc interactions. Briefly, lysates were subjected to immunoprecipitation (IP) with an anti-γc antibody. Immunoprecipitates were subjected to electrophoresis and the protein bands were transferred to PVDF membranes. Membranes corresponding to immunoprecipitated γc and total lysates (Tot.lys.) were probed with anti-γc or anti–IL-15Rα mAbs. The data presented are representative of three independent experiments. (B) Analysis of JAK3 phosphorylation by Western blotting. TF1 and TF1β cells were incubated with 10 ng/ml rIL-15 for 15 min at 37°C. Sister cultures were pretreated for 1 h with neutralizing anti–IL-15Rα, anti–GM-CSFRβ, and anti-gp130 mAbs. Cell extracts were subjected to immunoprecipitation with an anti-JAK3 mAb. JAK3 membranes were then re-probed with anti-phosphotyrosine 4G10 or anti-JAK3 mAbs. To correct for possible variations in the amount of protein loaded, values are expressed as pJAK3/JAK3 ratios. pJAK3/JAK3 levels were determined by densitometry, including correction for background (NIH Image software). Results are expressed as increases (e.g., two times) with respect to untreated cells. The data presented are representative of three independent experiments. (C) Coimmunoprecipitation. TF1 cells were analyzed for interactions between TRAF2, IL-15Rα, and γc. Briefly, lysates were subjected to immunoprecipitation (IP) with an anti-TRAF2 antibody. Immunoprecipitates were subjected to electrophoresis and the protein bands were transferred to PVDF membranes. Membranes corresponding to immunoprecipitated TRAF2 and total lysates (Tot.lys.) were probed with anti-TRAF2, anti-γc, or anti-IL-15Rα mAbs. The data presented are representative of three independent experiments. (D) Analysis of IκBα phosphorylation by Western blotting. TF1 and TF1β cells were incubated with 10 ng/ml rIL-15 or rIL-7 for 15 min at 37°C. Cell extracts were analyzed by Western blotting with anti-phospho-IκBα (pIκBα) antibody. Membranes were then reprobed with an antibody recognizing β-actin. To correct for possible variations in the amount of protein loaded, values are expressed as pIκBα/β actin ratios. pIκBα/β actin levels were determined by densitometry, including correction for background (NIH Image software). Results are expressed as increases with respect to untreated cells. The data presented are representative of three independent experiments.