Figure 2.
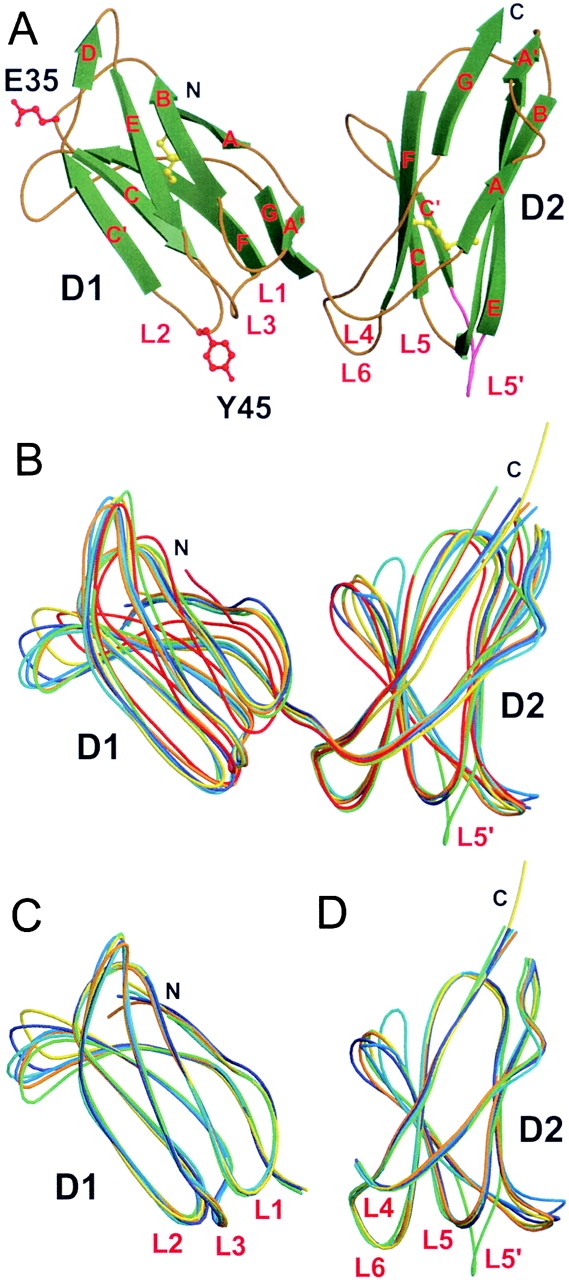
KIR2DS2 crystal structure and its comparison with other known KIR structures. (A) Overall structure of KIR2DS2 extracellular domains shown in molecular ribbon representation with the β-strands in green and the coils golden in color. The two amino acids different between KIR2DS2 and KIR2DL3 (within the sequence range 8–200), Tyr45(Y45) and Glu35(E35), are shown in red ball-and-stick representation. The L5′ loop between β-strands C′ and E of the D2 domain is represented in pink. The two cysteine disulfide bridges are shown in yellow ball-and-stick representation. Cα trace superpositions of (B) the complete KIR molecules (residues 8–200), (C) the isolated D1 domains (residues 8–102), and (D) the isolated D2 domains (residues 103–200) are represented as coils with different colors: KIR2DS2, green; KIR2DL1, red; KIR2DL2 (trigonal form), dark blue; KIR2DL2 (orthorhombic form), cyan; KIR2DL2 bound to HLA-Cw3 (KIR-A), orange; free KIR2DL2 (KIR-B) from the same KIR2DL2–HLA-Cw3 complex crystal, marine blue; and KIR2DL3, yellow.
