Abstract
The evolutionarily conserved, secreted protein Twisted gastrulation (Tsg) modulates morphogenetic effects of decapentaplegic (dpp) and its orthologs, the bone morphogenetic proteins 2 and 4 (BMP2/4), in early Drosophila and vertebrate embryos. We have uncovered a role for Tsg at a much later stage of mammalian development, during T cell differentiation in the thymus. BMP4 is expressed by thymic stroma and inhibits the proliferation of CD4−CD8− double-negative (DN) thymocytes and their differentiation to the CD4+CD8+ double-positive (DP) stage in vitro. Tsg is expressed by thymocytes and up-regulated after T cell receptor signaling at two developmental checkpoints, the transition from the DN to the DP and from the DP to the CD4+ or CD8+ single-positive stage. Tsg can synergize with the BMP inhibitor chordin to block the BMP4-mediated inhibition of thymocyte proliferation and differentiation. These data suggest that the developmentally regulated expression of Tsg may allow thymocytes to temporarily withdraw from inhibitory BMP signals.
Keywords: BMP4, Twisted gastrulation, thymocyte development, chordin, morphogen
Introduction
Thymocyte differentiation is controlled by signals via the pre-TCR and the TCR (for reviews, see references 1–3). Progression from the CD4−CD8− double-negative (DN)* to the CD4+CD8+ double-positive (DP) stage in cells destined for the TCR αβ lineage is driven by pre-TCR signaling and requires TCRβ chain rearrangement, expression, and pairing with the pre-TCR α chain. The acquisition of CD4 and CD8 is accompanied by a burst of proliferation, the cessation of further V to DJ rearrangement at the TCR β locus and proceeds through a transitional stage where (in the mouse strains used in this study) CD8 is expressed before CD4 (1). Recombination-deficient thymocytes are blocked at the CD4−CD8−CD25+CD44− stage of development but antibodies to the CD3ε signaling chain can mimic pre-TCR signaling and trigger progression to the DP stage (2). Further development from the DP to the CD4+ or CD8+ single-positive (SP) stage of development requires rearrangement and pairing of TCRα with TCRβ and the engagement of the resulting heterodimer with suitable peptide/MHC ligands on thymic stromal cells (3).
The control of cellular differentiation by antigen receptor rearrangement, expression, and engagement is unique to lymphocytes and (in evolutionary terms) a recent addition to older systems comprising soluble and cell-associated factors that control the survival, growth, and differentiation of cells and tissues in all multicellular organisms. For example, thymocyte survival and differentiation are promoted by cytokines including IL-7 (4), c-Kit ligand (5), IL-1, and TNF (6), and signaling through Notch (7) participates in T cell lineage commitment (8). Comparatively little is known about the role of patterning molecules or morphogens in lymphocyte development (9–12).
In a screen for genes that are regulated in a developmental stage-specific manner in thymocytes (13) we have identified Twisted gastrulation (Tsg; reference 14). Recent studies have revealed that the Tsg protein interacts with Drosophila decapentaplegic (dpp), the vertebrate dpp orthologs bone morphogenetic protein (BMP)2/4, and also the extracellular dpp/BMP inhibitors short gastrulation (sog)/chordin (15–20). In addition, Tsg can alter the proteolytic processing of sog/chordin by extracellular metalloproteases (16, 20). As a result, Tsg affects the binding of dpp/BMP2/4 to their cellular receptors and downstream signaling events mediated by the phosphorylation, nuclear translocation, and transcriptional activity of Smad proteins (for a review, see reference 21) positively (15, 20) or negatively (16–20). BMPs belong to a family of secreted signaling molecules the founding member of which, TGFβ, is essential for immune homeostasis (11, 22). In addition to a well-established role in embryonic patterning and development (23), BMP4 has been linked to hematopoesis: it specifies ventral mesoderm and blood cell formation in the Xenopus embryo (24), cooperates with VEGF to enhance hematopoetic cell generation from ES cells (25), is expressed in the human fetal AGM region (26), and regulates primitive human hematopoetic cell proliferation (27). There is evidence that components of the BMP signaling pathway are expressed in the thymus, including BMP4 itself (9), the extracellular BMP inhibitor chordin (28), the BMP receptor components activin-like kinase (ALK)-3 and -6 (BMPR1A and -B; reference 29), and Smad proteins (30, 31), the downstream mediators of BMP signaling. However, a role for BMP signals in thymocyte development has not been described. Furthermore, our understanding of Tsg is currently limited to early embryonic development (15–20, 32). We have investigated the effects of BMP4 and its modulation by Tsg during the transition from the CD4−CD8− DN to the CD4+CD8+ DP stage of thymocyte development.
Materials and Methods
Mouse Strains, Cell Sorting, Cell and Organ Culture.
Thymi were derived from wild-type (BALB/c or C57BL/6), recombination activating gene (Rag)1o/o (33), or Aβo/o β2mo/o (34, 35; referred to as MHCo/o in this manuscript). Where indicated, 3–4-wk-old Rag-1o/o mice were injected with 50 μg of the CD3ε mAb 2C11 (BD PharMingen). Thymocyte organ cultures and suspension cultures of mechanically dissociated or trypsinized fetal thymi were set up as hanging drops in inverted Terasaki plates (Nunc) in serum-free AIM-V lymphocyte medium (GIBCO BRL) supplemented with 2 × 10−5 M 2-ME, where indicated in the presence of recombinant BMP2, -4, and -7, chordin, neutralizing anti-BMP4 or BMPR-IA/Fc (all from R&D Systems), TGFβ1 (Sigma-Aldrich), or the CD3ε antibody 2C11 (BD PharMingen). Recombinant mouse Tsg was produced in X63 myeloma cells transfected with full-length mTsg cDNA (14) tagged with a COOH-terminal HA epitope and inserted into the BCMGS neo vector (36). Supernatant was concentrated, mTsg-HA captured with anti-HA–conjugated agarose beads (Sigma-Aldrich) and eluted by incubation of the washed beads with 100 μg/ml HA peptide (Sigma-Aldrich). Western analysis of the material with an anti-HA antibody (Sigma-Aldrich) showed a single band at ∼25 kD. Tsg concentration was estimated by gel staining since the HA peptide used for elution interfered with measurement of protein concentration. Supernatant from X63 cells transfected with empty BCMGS neo vector treated in the same way served as a control for mTsg-HA. All Tsg effects were confirmed using commercial mTsg (R&D Systems) which became available during the course of this study.
For some experiments thymocytes were stained with biotinylated antibodies to CD4 or CD8 and depleted with streptavidin-coated paramagnetic beads (Dynal). Thymic stromal cells were prepared by trypsinization of deoxyguanosine-treated fetal thymi and centrifugation over 55% Percoll (Amersham Pharmacia Biotech). Wild-type thymi were cultured for 18 to 72 h, thymus cell suspensions and thymocytes for 18 h, and Rag1o/o thymi for 48–72 h in the presence of the 2C11 mAb at 1 μg/ml. For phenotypic analysis on a FACSCalibur™ (Becton Dickinson) cells were counted and stained with CD4-PE or Cy-5 and CD8-FITC or -Cy5 (Caltag Laboratories), fixed for 10 min at room temperature in 0.2% paraformaldehyde, permeabilized with 0.2% Tween-20 for 15 min at 37°C, and incubated with 7-amino actinomycin D (7AAD). For sorting of DN and CD8 transitional cells on a FACS Vantage™ (Becton Dickinson), thymocytes were stained with CD4-Tricolor, CD8-PE (Caltag Laboratories), and heat stable antigen (HSA)-FITC.
Immunohistochemistry and RNA In Situ Hybridization on Frozen Sections.
Thymi from 3–4-wk-old C57BL/6 mice were snap frozen, 6-μm sections were prepared. For immunohistochemistry, sections were fixed in acetone and stained using polyclonal goat anti-BMP2/4 (R&D Systems) followed by horseradish peroxidase–conjugated rabbit anti–goat Ig antibody (Dako) and liquid DAB substrate-chromogen solution (Dako) according to the manufacturer's instructions. Slides were counterstained with Mayer's Hematoxylin (Sigma-Aldrich) and mounted in Kaiser's solution (14). Sense and antisense riboprobes were synthesized from a cloned PCR fragment spanning exon 4 of mTsg (14) in the presence of 11-dUTP digoxigenin (Roche) with T3 or T7 RNA polymerase. After hybridization, high-stringency washing, and RNase A digestion, the sections were incubated with alkaline phosphatase–conjugated anti-digoxigenin (Roche). BCIP/NBT (Roche) was used as substrate for detection. The sections were counterstained with nuclear fast red (Vector Laboratories), mounted in Mowiol solution (Calbiochem), and photographed.
Molecular Cloning and Analysis.
Expression studies were performed as reported previously (14, 37). For Northern blots, 1–5 μg total RNA were separated on 1% agarose gels containing formamide. The RNA was transferred to HybondN+ Nylon membranes and hybridized in 1.5× SSPE, 10% PEG 8000, 7% SDS at 68°C overnight. After washing in decreasing SSC concentrations and a final stringency wash in 0.1× SSC, 0.5% SDS at 60°C the membranes were exposed to BioMax-MS film (Eastman Kodak Co.).
For reverse transcription (RT)-PCR experiments, 0.3–1 μg total RNA were reverse transcribed with AMV reverse transcriptase (Promega) and amplified with the following primers at annealing temperatures of 60°C to 63°C: BMP2 no. 465 TACCGCAGGCACTCAGG, no. 466 CATTCCACCCCACATCACT; BMP4 no. 467 CGAGGCGACACTTCTACAG, no. 468 TGGGGGCTTCATAACCT; chordin: no. 473 CAAGCCTCAGCGGAAGAA, no. 474 CAAGCCCAGCCAATAGAACT; GAPDH: no. 251 TCTTCTTGTGCAGTGCC, no. 252 ACTCCACGACATACTCAGC. PCR products were analyzed on 2% agarose gels and the identity of the bands was confirmed by blotting and hybridizing with specific probes.
Western Blotting.
Phospho-Smad (pSmad)-2–specific antibodies were used to assess the phosphorylation status of Smad-2. Thymocyte lysates were separated (2 × 106 cell equivalents per lane) on 10% SDS polyacrylamide gels and blotted onto PVDF membranes (NEN Life Science Products). Blots were blocked (1% nonfat milk in PBS, 0.1% Tween 20 for p-Smad-2; 5% nonfat milk in PBS) and probed with rabbit anti-pSmad2 (Upstate Biotechnology 06–829) followed by alkaline phosphatase–conjugated anti–rabbit IgG and enhanced chemoluminescence detection (Western Star; Tropix) on MR-1 film (Eastman Kodak Co.). Blots were stripped and reprobed for total Smad-2 and subsequently for lamin B to control for equal loading and transfer efficiency (not shown).
Results
Tsg Up-regulation After TCR Signaling at Control Points in Thymocyte Development.
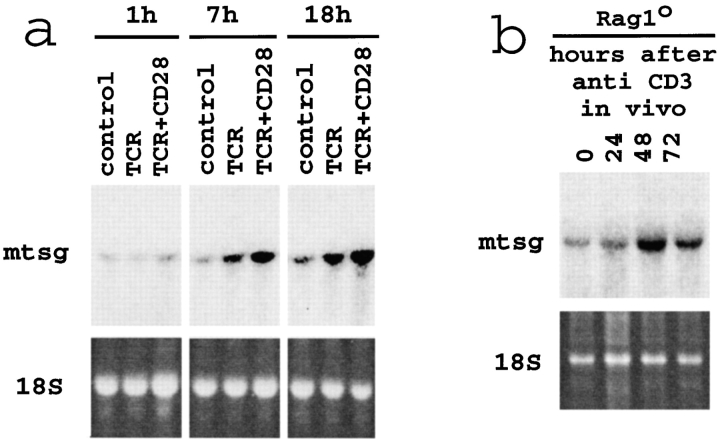
We undertook a screen to find novel genes involved in T cell development. MHC-naive CD4+CD8+ DP thymocytes (isolated from MHCo/o mice) were cocultured with thymic stromal cells derived from MHC-expressing (wild-type) or, as a control, from MHCo/o thymi (37). This system allowed a comparison of gene expression profiles between MHC-exposed and MHC-naive thymocytes by differential display PCR (13). The characterization of a band consistently up-regulated upon TCR signaling resulted in the cloning of the mouse homologue of Tsg (14). Northern blotting confirmed increased Tsg expression by MHC-naive DP thymocytes between 6 and 24 h after treatment with antibodies to the TCR alone or in combination with anti-CD28 (Fig. 1 a). To investigate Tsg expression at the pre-TCR–driven transition from the CD4−CD8− DN to the CD4+CD8+ DP stage we injected Rag1o/o mice with antibodies to CD3ɛ. Thymocyte development in Rag1o/o mice is blocked at the CD44−CD25+ DN stage of differentiation and can be rescued by CD3ε treatment in vivo which triggers a synchronous wave of differentiation from the CD44−CD25+ DN to the DP stage (2). This was accompanied by a rise in Tsg expression that occurred around 48 h after anti-CD3 injection (Fig. 1 b), at a time when thymocytes showed reduced CD25 expression and increased proliferation but were still DN (reference 2, and data not shown). We conclude that Tsg is differentially expressed after TCR signaling at two developmental checkpoints, the transition from the CD4−CD8− DN to the CD4+CD8+ DP and from the DP to the CD4+ or CD8+ SP stage.
Figure 1.
TCR-regulated Tsg expression by thymocytes. (a) Increased Tsg mRNA expression by MHC-naive DP thymocytes in response to antibodies to the TCR, with or without CD28 engagement. (b) Increased Tsg mRNA expression by DN thymocytes 48 h after injection of Rag-1o/o mice with anti-CD3ε. In additional experiments, Tsg upregulation occurred between 24 and 72 h and persisted until 120 h after injection.
The recognized role of Tsg as a modulator of BMP signaling (15–20) prompted us to reevaluate the expression of components of the BMP signaling pathway in the thymus (see Introduction). RT-PCR showed the expression of BMP2, BMP4, BMP7, and chordin in total thymus tissue (Fig. 2 a). Analysis of separated thymocytes and thymic stroma established thymic stromal cells as the source of BMP2/4 and chordin. BMP7 was expressed by both thymocytes and stromal cells (Fig. 2 a). Semiquantitative RT-PCR analysis confirmed that thymocytes expressed BMP7 at levels similar to total thymus whereas the levels of BMP4 and chordin expression in thymocytes were 20- to 100-fold lower in thymocytes than total thymus (data not shown). The distribution of BMP2/4 protein in the thymus was visualized by immunohistochemistry on frozen sections (Fig. 2, b–e). We noted marked regional differences with staining predominantly of subcapsular (sc) and medullary (med) regions. Within these areas the signal appeared ‘patchy’ rather than uniform (Fig. 2, c and e). In situ hybridization showed Tsg expressing cells distributed throughout the cortex and the medulla (Fig. 2, f–h).
Figure 2.
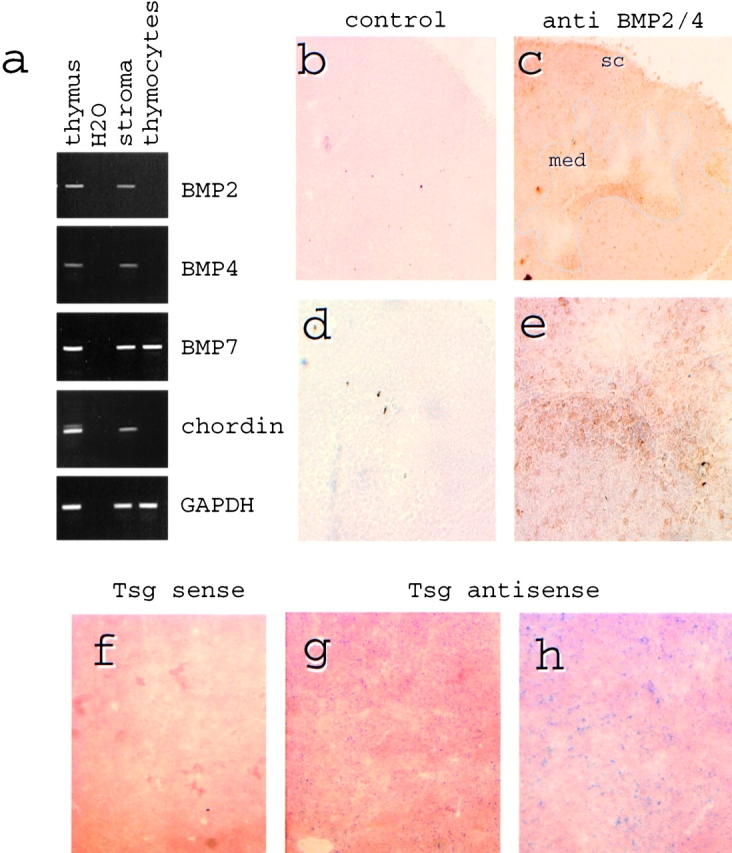
Expression and distribution of intrathymic BMPs and chordin. (a) Total thymus or isolated stromal cells and thymocytes were subjected to RT-PCR analysis to establish the sources of BMP2, BMP4, BMP7, and chordin in the thymus. GAPDH served as a positive control. H2O lanes contained no cDNA. (b–e) Immunoperoxidase staining of normal 3–4-wk-old mouse (C57BL/6) thymus with goat anti-BMP/24 (c and e) or control goat antibody (b and d). BMP2/4 staining (brown) is seen mainly within supcapsular (sc) and medullary (med, outlined in c) regions (original magnification: ×200, b and c) and ‘patchy’ within positive areas (original magnification: ×400, d and e). (f–h) In situ hybridization of normal 3–4-wk-old mouse (C57BL/6) thymus with Tsg sense (f) and antisense (g and h) probes. Tsg expressing cells are distributed throughout the cortex and the medulla. Original magnification: ×100 (f and g), ×200 (h).
BMP4 Can Interfere with Cell Cycle Activity and Developmental Progression of DN Thymocytes.
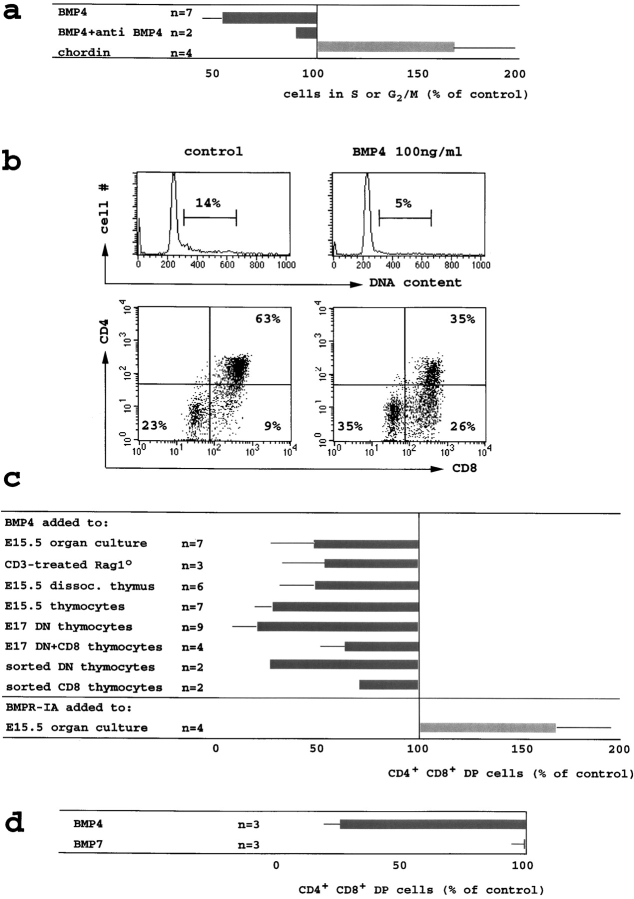
To assess the functional effects of BMPs we established thymic organ cultures at embryonic day 15.5 (E15.5), a time in ontogeny when all thymocytes are DN, and analyzed the cell cycle distribution as well as the phenotype of thymocytes that developed in these cultures. Addition of BMP4 at 100 ng/ml (∼6 nM) reduced the percentage of thymocytes in S and G2/M phase of the cell cycle by 42 ± 9% (Fig. 3 , a and b). This inhibition was largely abrogated by a neutralizing antibody to BMP4 (Fig. 3 a). Conversely, treatment of thymic organ cultures with recombinant chordin 2 μg/ml (∼20 nM) increased the percentage of thymocytes in S and G2/M by 59 ± 30% (Fig. 3 a). As chordin is an extracellular inhibitor specifically of BMPs but not of TGFβ or activin (21), the increased proliferation of thymocytes in intact thymic lobes treated with chordin suggests that thymocyte cell cycle activity is subject to BMP-mediated inhibition in situ.
Figure 3.
BMP4 inhibits the cell cycle activity and the developmental progression of DN thymocytes to the DP stage. (a) E15.5 thymic lobes were cultured in hanging drops for 24–36 h with BMP4 (100 ng/ml, 6 nM approximately), BMP4 (100 ng/ml) plus neutralizing anti-BMP4 (10 μg/ml) or chordin (2 μg/ml, 20 nM approximately). Thymocytes were stained for CD4 and CD8, fixed, permeabilized, and DNA content was visualized by 7AAD. The fraction of cells with a DNA content >G1 is shown relative to control cultures (16 ± 7% >G1). (b) Reduced cell cycle activity and impaired developmental progression in BMP4-treated E15.5 thymic organ cultures (36 h, methods as in a). (c) BMP4 (100 ng/ml) inhibited the DN to DP transition in E15.5 thymic organ cultures by 52 ± 21% (from 50 ± 10% DP to 25 ± 13% at 36 h, top row) and in Rag1o/o thymic organ cultures treated for 72 h with anti-CD3ε (1 μg/ml) by 46 ± 22% (from 57 ± 4% DP to 32 ± 12%, row two). BMP4 reduced the generation of DP cells in proteolytically dissociated E15.5 thymus suspensions by 52 ± 17% (from 59 ± 13% DP to 28 ± 9%, third row), in mechanically prepared E15.5 thymocyte suspensions by 72 ± 8% (from 44 ± 19% DP to 13 ± 6%, row four), in DN cells prepared by CD8 depletion of E17 thymocytes by 80 ± 13% (from 33 ± 16% to 8 ± 8%, row five), in DN/CD8 transitional thymocytes prepared by CD4 depletion of E17 thymocytes by 36 ± 10% (from 66 ± 23% DP to 44 ± 20%, row six), in highly purified DN by 72% (from 39% DP to 11%) and 73% (from 40% DP to 10%) in two experiments (row seven), and in highly purified CD8 transitional thymocytes by 26% (from 91% DP to 67%) and 29%, (from 83% DP to 59%) in two experiments (row eight, all suspension culture experiments read out at 18 h). Recombinant BMPR-IA/Fc (bottom row, 1–3 μg/ml) increased the generation of DP cells in E15.5 thymic lobes cultured for 18 h by 67 ± 28% (from 23 to 38%, n = 4). (d) E15.5 thymus suspensions were cultured with 300 ng/ml of BMP4 or BMP7 and analyzed as in Fig. 3, b and c. BMP4 reduced the generation of DP cells by 73 ± 7% (from 50 ± 27% DP to 14 ± 9%) but BMP7 did not (1 ± 5%).
In addition to proliferation, BMP4 treatment of E15.5 thymic organ cultures interfered with the developmental progression from the CD4−CD8− DN to the CD4+CD8+ DP stage (Fig. 3 b) and reduced the generation of DP cells on average by 52 ± 21% (Fig. 3 c). In control experiments, the survival of sorted E17 DP thymocytes was unaffected by overnight culture in BMP4 (100 ng/ml), indicating that BMP4 was not simply toxic to DP thymocytes (data not shown).
The reduced generation of DP thymocytes in the presence of BMP4 could be explained either by a delay in TCRβ chain rearrangement or by a reduced response to pre-TCR signaling. To address this issue we assayed BMP4 effects on the differentiation of Rag1-deficient thymocytes in response to CD3ε antibody (1 μg/ml) as a surrogate signal which does not require TCRβ chain rearrangement. BMP4 treatment reduced the development of DP thymocytes in Rag1o/o organ cultures treated with CD3ε antibodies by 46 ± 22% (Fig. 3 c).
To ask whether BMP4 inhibition of the DN to DP transition required an intact thymic microenvironment, E15.5 thymi were disrupted by trypsinization to yield suspensions containing all thymic cell types including thymic stroma. BMP4 blocked the DN to DP transition by 52 ± 17%. Similarly, BMP4 blocked the DN to DP transition in mechanically prepared E15.5 thymocyte suspensions by 72 ± 8% (Fig. 3 c).
The ability of BMP4 to inhibit thymocyte differentiation in suspension cultures allowed us to define the BMP4 sensitivity of thymocytes at different developmental stages and, in addition, to ask whether BMP4 acts directly on thymocytes or via intermediate cell types. When E17 thymocyte suspensions were depleted of CD8+ thymocytes to yield pure DN cells, BMP4 inhibited the generation of DP cells by 80 ± 13%. In contrast, when E17 thymocyte suspensions were depleted only of CD4+ thymocytes, leaving behind DN as well as CD8 transitional cells, BMP4 was less effective at inhibiting the generation of DP cells (36 ± 10%). Hence, DN thymocytes were more sensitive to BMP4 than CD8 transitional cells (Fig. 3 c).
To determine whether BMP4 affects thymocyte development directly or indirectly, DN thymocytes were highly purified by two rounds of cell sorting for HSAhighCD4−CD8− thymocytes, yielding populations that contained 0.3% or less of cell types other than thymocytes. Sorted thymocytes differentiated efficiently to the DP stage and BMP4 inhibited this by 72% and 73% in two independent experiments. The developmental progression of highly purified CD8 transitional cells was blocked by BMP4 to a lesser degree, by 26 and 29% in two independent experiments (Fig. 3 c). Hence BMP4 acts directly on DN thymocytes to inhibit their progression to the DP stage.
We exploited the observation that BMP2 and 4 (and to a lesser extent BMP7) but not TGFβ or activin can bind BMPR-IA with high affinity in the absence of type II receptor subunits (38, 39) to address whether the DN to DP transition is affected by endogenous BMPs. Soluble BMP receptor-IA subunit (BMPR-IA) was added to intact E15.5 thymic lobes in organ culture. In the presence of BMPR-IA, the percentage of DP thymocytes (analyzed 18 h later instead of 24–36 h in the organ culture experiments described above) was elevated by 67 ± 28% relative to control thymi (Fig. 3 c) and the number of DP cells per thymic lobe increased by 94 ± 57% (not shown).
Exogenous BMP2 blocked the DN to DP transition to the same extent as BMP4 (not shown). In contrast, BMP7 did not inhibit the DN to DP transition (Fig. 3 d), even at very high concentrations (1,000 ng/ml, not shown).
Smad-2 Phosphorylation in Response to TGFβ but Not BMP4 in Thymocytes.
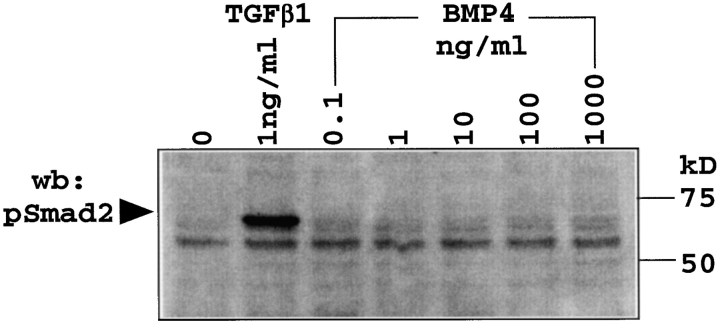
Like BMP4, TGFβ blocks thymocyte cell cycle activity and developmental progression (10, 40). To address the question whether thymocytes can distinguish BMP4 from TGFβ signals we analyzed the phosphorylation status of Smad-2, a downstream mediator of TGFβ signals (21) in thymocytes cultured with TGFβ1 at 1 ng/ml or a range of BMP4 concentrations from 0.1 to 1,000 ng/ml. Phosphorylated Smad-2 was readily detected in response to TGFβ but not to BMP4 by Western blotting with pSmad-2–specific antibodies (Fig. 4) . Hence, BMP4 signaling does not appear to utilize the canonical TGFβ signaling pathway in thymocytes. An analysis of Smad-1 phosphorylation in response to exogenous BMP4 remained inconclusive as pSmad-1 appeared to be present in freshly isolated thymocytes (not shown, and see Discussion).
Figure 4.
TGFβ but not BMP4 induce Smad-2 phosphorylation in thymocytes. Thymocytes from E17 (or postnatal, not shown) wild-type (C57BL/6) mice were cultured with TGFβ1 (1 ng/ml) or the indicated concentrations of BMP4 for 45 min and cell lysates assayed for pSmad-2 by Western blot.
Tsg Synergizes with the BMP Inhibitor Chordin to Block BMP4 Effects on Thymocyte Differentiation.
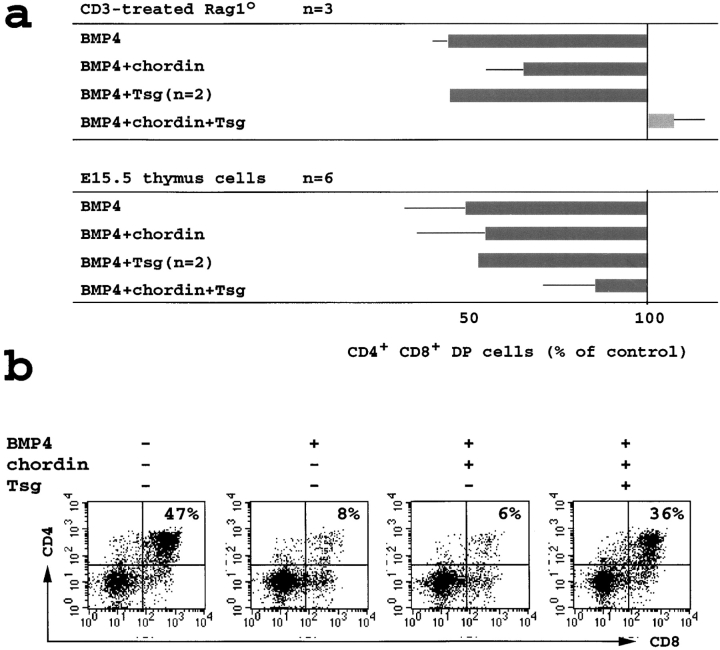
As described in Fig. 3 c, BMP4 inhibited the developmental progression from the DN to DP stage in Rag1o/o organ cultures treated with CD3ε. In the presence of BMP4 (100 ng/ml) the BMP inhibitor chordin restored the generation of DP cells only partially, even when added in threefold molar excess (2 μg/ml). We used this system to assess the functional effects of Tsg. On its own (not shown) or in combination with BMP4 (100 ng/ml), Tsg (1 μg/ml, ∼40 nM) had little effect. In combination with chordin, however, TSG was able to reverse the inhibitory effects of BMP4 on the generation of DP thymocytes in anti-CD3ε–treated Rag1o/o organ cultures (Fig. 5 a, top panel). Rescue of DP differentiation from BMP4-mediated inhibition by chordin and Tsg was also seen in trypsinized suspension cultures of E15.5 thymi. Again, chordin antagonized BMP4 only partially, Tsg on its own was without effect, but the combination of chordin and Tsg rescued DP thymocyte development effectively (Fig. 5 a, bottom panel). A representative experiment is shown in Fig. 5 b.
Figure 5.
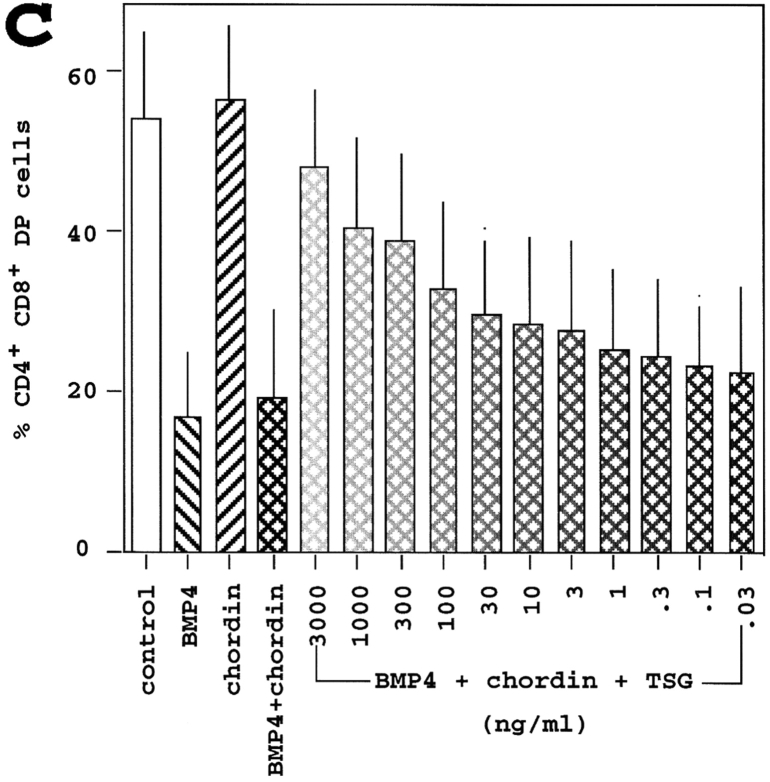
Tsg synergizes with chordin to block the effects of exogenous BMP4 on thymocyte developmental progression. (a) Top panel; Rag1o/o organ cultures were treated with the anti-CD3ε antibody 2C11 for 3 d (1 μg/ml) with the addition of BMP4 (100 ng/ml), chordin (2 μg/ml), and/or Tsg (1 μg/ml). Thymocytes were analyzed as in Fig. 3. Note the restoration of DP thymocyte development by the combination of chordin and Tsg. Bottom panel; suspension cultures of wild-type E15.5 thymi cultured overnight with the same additions as in top panel. (b) Suspension cultures of wild-type E15.5 thymi were cultured overnight and analyzed as in a. (c) E15.5 thymus suspensions were treated with BMP4 (100 ng/ml, left diagonal pattern), chordin (2 μg/ml, right diagonal pattern), or BMP4 plus chordin (cross-hatched) with or without Tsg (0.03 to 3,000 ng/ml) and analyzed as in Fig. 3. The percentage of DP thymocytes generated after 24 h is given (mean ± SD, n = 4).
The ratio between chordin and Tsg was found critical for BMP antagonism read out as secondary axis formation in zebrafish (19). We therefore performed experiments in which the concentrations of BMP4 (100 ng/ml) and chordin (2 μg/ml) were kept constant and Tsg was titrated over a wide range of concentrations, from 0.03 to 3,000 ng/ml (Fig. 5 b). Tsg addition resulted in a dose-dependent inhibition of the BMP4 block on the DN to DP transition. We conclude that Tsg can synergize with chordin to antagonize BMP4-mediated inhibition of the DN to DP transition. In this system there was no indication that Tsg had BMP-agonistic effects at any ratio of chordin and Tsg tested.
Discussion
The key observation of our study is that immature thymocytes express Tsg, an extracellular modifier of BMP2/4 activity, in a developmentally regulated fashion: pre-TCR and TCR signaling results in increased Tsg expression at two developmental checkpoints, the DN to DP and the DP to SP transition (Fig. 1). Tsg has recently been shown to interact with extracellular components of the BMP signaling pathway (15–20), prompting us to investigate effects of BMP4 on thymocyte development. We found that DN thymocytes are highly susceptible to BMP4, which acts directly and without relay by another cell type to reduce DN thymocyte proliferation and progression to the DP stage in vitro (Fig. 3 c). The thymocyte response to BMP4 appears not to utilize the canonical TGFβ signaling cascade as indicated by the phosphorylation of Smad-2 in response to TGFβ but not to BMP4 (Fig. 4).
The addition of specific BMP inhibitors to intact thymic lobes results in elevated rates of thymocyte proliferation (Fig. 3 a) and accelerated pre-TCR dependent differentiation to the DP stage (Fig. 3 c), indicating that a BMP-imposed ‘brake’ may be active in situ. Conversely, exogenous BMP4 interferes with DN proliferation progression to the DP stage (Fig. 3, a–d), suggesting that endogenous BMP2/4 levels are subsaturating, or, alternatively, that not all thymocytes are exposed to equal BMP2/4 levels in situ. The latter possibility is consistent with our analysis of thymic sections where (in contrast to the uniform expression of BMP in early embryos; for a review, see reference 23) we found marked regional differences: BMP2/4 was seen predominantly in subcapsular and medullary areas and showed an uneven, ‘patchy’ distribution within these areas (Fig. 2, c and e). By analogy to the Drosophila ovary where the BMP2/4 ortholog dpp serves to retain germ cell precursors in a concentration-dependent fashion (41) one might speculate that BMP2/4-rich areas represent specialized microenvironments geared toward the retention of precursor cells. In contrast to BMP2/4, the expression of BMP7 (which together with BMP5 and -6 forms the 60A subgroup of BMPs distinct from BMP2/4; reference 23) is not restricted to thymic stroma and BMP7 is expressed abundantly in thymocytes (Fig. 2 a). Interestingly, exogenous BMP7 did not inhibit the DN to DP transition (Fig. 3 d). Differences in the response to BMP2/4 and 7 have previously been seen in developing neurons (42, 43). Both BMP2/4 and BMP7 signaling involve Smad-1 (21, 23) and it is unclear how developing neurons (42, 43) or thymocytes (this study) discriminate between these BMPs. BMP7 may contribute to pSmad-1 seen in freshly isolated thymocytes (data not shown). In addition to BMP2/4 (this paper), TGFβ1 and the morphogen sonic hedgehog (Shh) can block the pre-TCR–dependent transition from the DN to the DP stage in vitro (9, 40). The relationship between BMP, Shh, and Wnt signals in the thymus remains to be defined. In certain developmental contexts Shh acts upstream of dpp/BMP2/4 (27, 41, 44–47). Conversely, Wnt and its downstream effectors (which can promote thymocyte differentiation; references 11 and 12) have been shown to block BMP4 expression in Xenopus embryos (48).
Reportedly, Tsg can either facilitate or antagonize dpp/BMP2/4 activity (15–20), perhaps because the release of dpp/BMP2/4 from the extracellular inhibitors sog/chordin is alternatively blocked (16) or facilitated (20) by the proteolytic processing of sog/chordin by metalloproteases of the tolloid/BMP1 family. The relative abundance of Tsg and chordin can be critical for whether Tsg acts as an agonist or an antagonist of dpp/BMP2/4 (17). In our own experiments Tsg synergized with chordin to antagonize BMP4 in a simple, dose-dependent manner (Fig. 5). If BMP2/4 originating from thymic stroma can block the expansion and differentiation of DN thymocytes in vivo, the developmentally regulated expression of Tsg could serve to temporarily antagonize inhibitory BMP effects following successful TCRβ rearrangement and pre-TCR expression (Fig. 6) . We speculate that the balance between BMP2/4, chordin, and Tsg may ensure developmental progression while maintaining a sufficient pool of immature precursors. Although these ideas are consistent with our data on the developmental regulation of Tsg expression in vivo and our experiments in vitro, we emphasize that BMPs may have additional functions in the thymus. For example, a role at the DP stage is implied by differential Tsg expression in response to TCR engagement at the DP stage (Fig. 1) and the recently reported failure of thymocyte differentiate to the SP stage in the absence of Schnurri2, a putative downstream target of BMP signaling (49). Moreover, BMP4 and dpp act as morphogens in vertebrate and Drosophila embryos where they form activity gradients to specify distinct cell fates along the dorsal/ventral axis (23, 50–53). It will therefore be of interest to find out whether local BMP2/4 concentration specifies alternative fates such as αβ versus γδ at the DN to DP transition or CD4 versus CD8 at the DP to SP transition.
Figure 6.
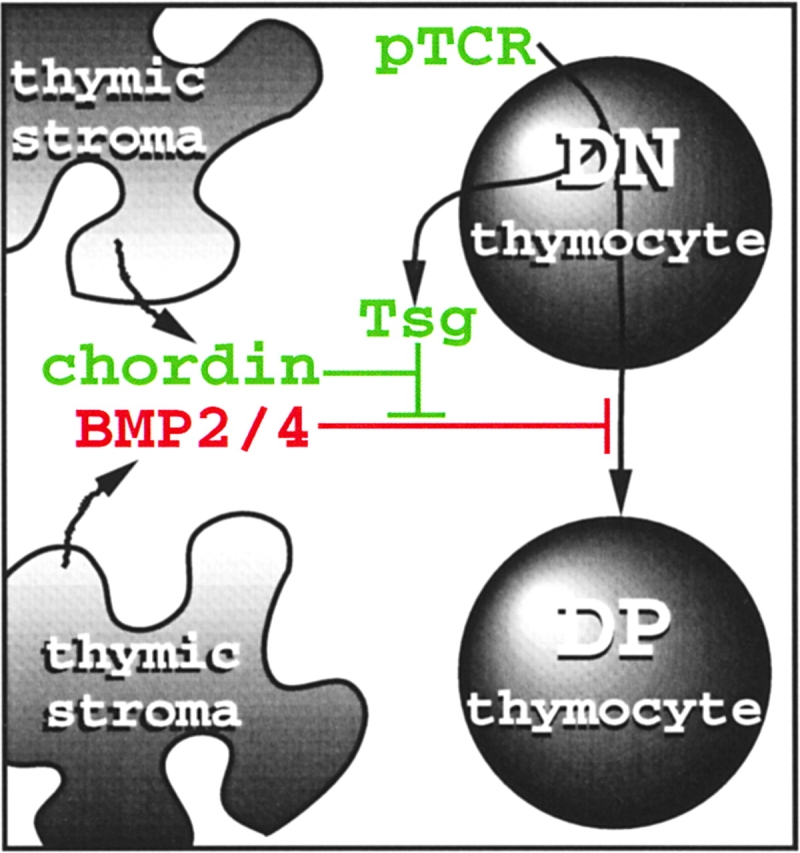
How BMP4, chordin, and Tsg may affect the pre-TCR–dependent DN to DP transition (see text for details).
Our finding of developmentally regulated Tsg expression in the thymus extends the concept that cells within a morphogenetic field not only read and respond to the local morphogen concentration but can be instrumental in shaping the morphogen gradient (52, 53). It suggests that cells can temporarily withdraw from signaling molecules affecting their differentiation via the increased expression of a secreted modifier at specific developmental control points.
Acknowledgments
We thank Drs. Les Dale and Peter ten Dijke for discussions, constructs, and antibodies, Jonathan Carter for help with the mTsg-HA construct, Katy Smith for cell sorting, Spiros Lalos for help with histochemistry, and Dimitris Kontoyannis for comments on the manuscript.
Supported by the Medical Research Council, UK. D. Graf is currently the recipient of a Marie Curie Fellowship and would like to thank George Kollias for encouragement and support. D.B. Palmer is the recipient of a Medical Research Council Career Development Award.
Footnotes
Abbreviations used in this paper: BMP, bone morphogenetic protein; DN, double negative; DP, double positive; dpp, decapentaplegic; pSmad, phospho-Smad; Rag, recombination activating gene; RT, reverse transcription; sog, short gastrulation; SP, single positive; Tsg, twisted gastrulation.
References
- 1.Kisielow, P., and H. von Boehmer. 1995. Development and selection of T cells: facts and puzzles. Adv. Immunol. 58:87–209. [DOI] [PubMed] [Google Scholar]
- 2.Levelt, C.N., and K. Eichmann. 1995. Receptors and signals in early thymic selection. Immunity. 3:667–672. [DOI] [PubMed] [Google Scholar]
- 3.Jameson, S.C., K.A. Hogquist, and M.J. Bevan. 1995. Positive selection of thymocytes. Annu. Rev. Immunol. 13:93–126. [DOI] [PubMed] [Google Scholar]
- 4.Peschon, J.J., P.J. Morrissey, K.H. Grabstein, F.J. Ramsdell, E. Maraskovsky, B.C. Gliniak, L.S. Park, S.F. Ziegler, and D.E. Williams, C.B. Ware, et al. 1994. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 180:1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodewald, H.R., M. Ogawa, C. Haller, C. Waskow, and J.P. DiSanto. 1997. Pro-thymocyte expansion by c-kit and the common cytokine receptor gamma chain is essential for repertoire formation. Immunity. 6:265–272. [DOI] [PubMed] [Google Scholar]
- 6.Zúñiga-Pflücker, J.C., D. Jiang, and M.J. Lenardo. 1995. Requirement for TNF- and IL-1 in fetal thymocyte commitment and differentiation. Science. 268:1906–1909. [DOI] [PubMed] [Google Scholar]
- 7.Simpson, P. 1997. Notch signaling in development. Perspect. Dev. Neurobiol. 4:297–304. [PubMed] [Google Scholar]
- 8.MacDonald, H.R., A. Wilson, and F. Radtke. 2001. Notch1 and T-cell development: insights from conditional knockout mice. Trends Immunol. 22:155–160. [DOI] [PubMed] [Google Scholar]
- 9.Outram, S.V., A. Varas, C.V. Pepicelli, and T. Crompton. 2000. Hedgehog signaling regulates differentiation from double-negative to double-positive thymocyte. Immunity. 13:187–197. [DOI] [PubMed] [Google Scholar]
- 10.Letterio, J.L., and A.B. Roberts. 1998. Regulation of immune responses by TGF-β. Annu. Rev. Immunol. 16:137–161. [DOI] [PubMed] [Google Scholar]
- 11.Staal, F.J., J. Meeldijk, P. Moerer, P. Jay, B.C. van de Weerdt, S. Vainio, G.P. Nolan, and H. Clevers. 2001. Wnt signaling is required for thymocyte development and activates Tcf-1 mediated transcription. Eur. J. Immunol. 31:285–293. [DOI] [PubMed] [Google Scholar]
- 12.Gounari, F., I. Aifantis, K. Khazaie, S. Hoeflinger, N. Harada, M.M. Taketo, and H. von Boehmer. 2001. Somatic activation of beta-catenin bypasses pre-TCR signaling and TCR selection in thymocyte development. Nat. Immunol. 2:863–869. [DOI] [PubMed] [Google Scholar]
- 13.Graf, D., A.G. Fisher, and M. Merkenschlager. 1997. Rational primer design greatly improves differential display-PCR (DD-PCR). Nucleic Acids Res. 25:2239–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graf, D., P.M. Timmons, M. Hitchins, V. Episkopou, G. Moore, T. Ito, A. Fujiyama, A.G. Fisher, and M. Merkenschlager. 2001. Evolutionary conservation, developmental expression, and genomic mapping of mammalian Twisted gastrulation. Mamm. Genome. 12:554–560. [DOI] [PubMed] [Google Scholar]
- 15.Oelgeschläger, M., J. Larrain, D. Geissert, and E.M. De Robertis. 2000. The evolutionary conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature. 405:757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu, K., S. Srinivasan, O. Shimmi, B. Biehs, K.E. Rashka, D. Kimelman, M.B. O'Connor, and E. Bier. 2000. Processing of the Drosophila Sog protein creates a novel BMP inhibitory activity. Development. 127:2143–2154. [DOI] [PubMed] [Google Scholar]
- 17.Scott, I.C., I.L. Blitz, WN. Pappano, S.A. Maas, KW. Cho, and D.S. Greenspan. 2001. Homologues of Twisted gastrulation are extracellular cofactors in antagonism of BMP signalling. Nature. 410:475–478. [DOI] [PubMed] [Google Scholar]
- 18.Chang, C., D.A. Holtzman, S. Chau, T. Chickering, E.A. Woolf, LM. Holmgren, J. Bodorova, D.P. Gearing, W.E. Holmes, and A.H. Brivanlou. 2001. Twisted gastrulation can function as a BMP antagonist. Nature. 410:483–487. [DOI] [PubMed] [Google Scholar]
- 19.Ross, J.J., O. Shimmi, P. Vilmos, A. Petryk, H. Kim, K. Gaudenz, S. Hermanson, S.C. Ekker, M.B. O'Connor, and J.L. Marsh. 2001. Twisted gastrulation is a conserved extracellular BMP antagonist. Nature. 410:479–483. [DOI] [PubMed] [Google Scholar]
- 20.Larrain, J., M. Oelgeschlager, N.I. Ketpura, B. Reversade, L. Zakin, and E.M. De Robertis. 2001. Proteolytic cleavage of Chordin as a switch for the dual activities of Twisted gastrulation in BMP signaling. Development. 128:4439–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massague, J. 1998. TGFβ signal transduction. Annu. Rev. Biochem. 67:753–791. [DOI] [PubMed] [Google Scholar]
- 22.Gorelik, L., and R.A. Flavell. 2000. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 12:171–181. [DOI] [PubMed] [Google Scholar]
- 23.Hogan, B.L.M. 1996. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 10:1580–1594. [DOI] [PubMed] [Google Scholar]
- 24.Huber, T.L., and L.I. Zon. 1998. Transcriptional regulation of blood formation during Xenopus development. Semin. Immunol. 10:103–109. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama, N., J. Lee, and L. Chiu. 2000. Vascular endothelial growth factor synergistically enhances bone morphogenetic protein-4-dependent lymphohematopoietic cell generation from embryonic stem cells in vitro. Blood. 95:2275–2283. [PubMed] [Google Scholar]
- 26.Marshall, C.J., C. Kinnon, and A.J. Thrasher. 2000. Polarized expression of bone morpho-genetic protein-4 in the human aorta-gonad-mesonephros region. Blood. 96:1591–1593. [PubMed] [Google Scholar]
- 27.Bhardwaj, G., B. Murdoch, D. Wu, D.P. Baker, K.P. Williams, K. Chadwick, L.E. Ling, F.N. Karanu, and M. Bhatia. 2001. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat. Immunol. 2:172–180. [DOI] [PubMed] [Google Scholar]
- 28.Scott, I.C., B.M. Steiglitz, T.G. Clark, W.N. Pappano, and D.S Greenspan. 2000. Spatiotemporal expression patterns of mammalian chordin during postgastrulation embryogenesis and in postnatal brain. Dev. Dyn. 217:449–456. [DOI] [PubMed] [Google Scholar]
- 29.Dewulf, N., K. Verschueren, O. Lonnoy, A. Moren, S. Grimsby, K. Vande Spiegle, K. Miyazono, D. Huylebroeck, and P. Ten Dijke. 1995. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 136:2653–2663. [DOI] [PubMed] [Google Scholar]
- 30.Dick, A., W. Risau, and H. Drexler. 1998. Expression of Smad1 and Smad2 during embryogenesis suggests a role in organ development. Dev. Dyn. 211:293–305. [DOI] [PubMed] [Google Scholar]
- 31.Flanders, K.C., E.S. Kim, and A.B. Roberts. 2001. Immunohistochemical expression of Smads 1-6 in the 15-day gestation mouse embryo: signaling by BMPs and TGF-betas. Dev. Dyn. 220:141–154. [DOI] [PubMed] [Google Scholar]
- 32.Mason, E.D., K.D. Konrad, C.D. Webb, and J.L. Marsh. 1994. Dorsal midline fate in drosophila embryos requires twisted gastrulation, a gene encoding a secreted protein related to human connective tissue growth factor. Genes Dev. 8:1489–1501. [DOI] [PubMed] [Google Scholar]
- 33.Spanopoulou, E., P. Cortes, C. Shih, C.M. Huang, D.P. Silver, P. Svec, and D. Baltimore. 1995. Localization, interaction, and RNA binding properties of the V(D)J recombination-activating proteins RAG1 and RAG2. Immunity. 3:715–726. [DOI] [PubMed] [Google Scholar]
- 34.Koller, B.H., P. Marrack, J.W. Kappler, and O. Smithies. 1990. Normal development of mice deficient in beta 2m, MHC class I proteins, and CD8+ T cells. Science. 248:1227–1230. [DOI] [PubMed] [Google Scholar]
- 35.Cosgrove, D., D. Gray, A. Dierich, J. Kaufman, M. Lemeur, C. Benoist, and D. Mathis. 1991. Mice lacking MHC class II molecules. Cell. 66:1051–1066. [DOI] [PubMed] [Google Scholar]
- 36.Karasuyama, H., and F. Melchers. 1988. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur. J. Immunol. 18:97–104. [DOI] [PubMed] [Google Scholar]
- 37.Merkenschlager, M., D. Graf, M. Lovatt, U. Bommhardt, R. Zamoyska, and A.G. Fisher. 1997. How many thymocytes audition for selection? J. Exp. Med. 186:1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natsume, T., S. Tomita, S. Iemura, N. Kinto, A. Yamaguchi and N. Ueno. 1997. Interaction between soluble type I receptor for bone morphogenetic protein and bone morphogenetic protein-4. J. Biol. Chem. 272:11535–11540. [DOI] [PubMed] [Google Scholar]
- 39.Kawabata, M., T. Imamura, and K. Miyazono. 1998. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 9:49–61. [DOI] [PubMed] [Google Scholar]
- 40.Takahama, Y., J.J. Letterio, H. Suzuki, A.G. Farr, and A. Singer. 1994. Early progression of thymocytes along the CD4/CD8 developmental pathway is regulated by a subset of thymic epithelial cells expressing transforming growth factor beta. J. Exp. Med. 179:1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie, T., and A.C. Spradling. 1998. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 94:251–260. [DOI] [PubMed] [Google Scholar]
- 42.Furuta, Y., D.W. Piston, and B.L.M. Hogan. 1997. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 124:2203–2212. [DOI] [PubMed] [Google Scholar]
- 43.Shou, J., R.C. Murray, P.C. Rim, and A.L. Calof. 2000. Opposing effects of bone morphogenetic proteins on neuron production and survival in the olfactory receptor neuron lineage. Development. 127:5403–5413. [DOI] [PubMed] [Google Scholar]
- 44.Xie, T., and A.C. Spradling. 2000. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 290:328–330. [DOI] [PubMed] [Google Scholar]
- 45.Laufer, E., C.E. Nelson, R.L. Johnson, B.A. Morgan, and C. Tabin. 1994. Sonic hedgehog and Fgf-4 act through a signaling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell. 79:993–1003. [DOI] [PubMed] [Google Scholar]
- 46.Murtaugh, L.C., J.H. Chyung, and A.B. Lassar. 1999. Sonic hedgehog promotes somitic chondrogenesis by altering the cellular response to BMP signaling. Genes Dev. 13:225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zuniga, A., A.P. Haramis, A.P. McMahon, and R. Zeller. 1999. Signal relay by BMP antagonism controls the SHH/FGF4 feedback loop in vertebrate limb buds. Nature. 401:598–602. [DOI] [PubMed] [Google Scholar]
- 48.Baker, J.C., R.S. Beddington, and R.M. Harland. 1999. Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev. 13:3149–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takagi, T., J. Harada, and S. Ishii. 2001. Murine Schnurri-2 is required for positive selection of thymocytes. Nat. Immunol. 2:1048–1053. [DOI] [PubMed] [Google Scholar]
- 50.Gurdon, J.B., S. Dyson, and D. St. Johnston. 1998. Cells' perception of position in a concentration gradient. Cell. 95:159–162. [DOI] [PubMed] [Google Scholar]
- 51.Dale, L. 2000. Pattern formation: a new twist to BMP signalling. Curr. Biol. 10:R671–R673. [DOI] [PubMed] [Google Scholar]
- 52.Tabata, T. 2001. Genetics of morphogen gradients. Nat. Rev. Genet. 2:620–630. [DOI] [PubMed] [Google Scholar]
- 53.Teleman, A.A., M. Strigini, and S.M. Cohen. 2001. Shaping morphogen gradients. Cell. 105:559–562. [DOI] [PubMed] [Google Scholar]