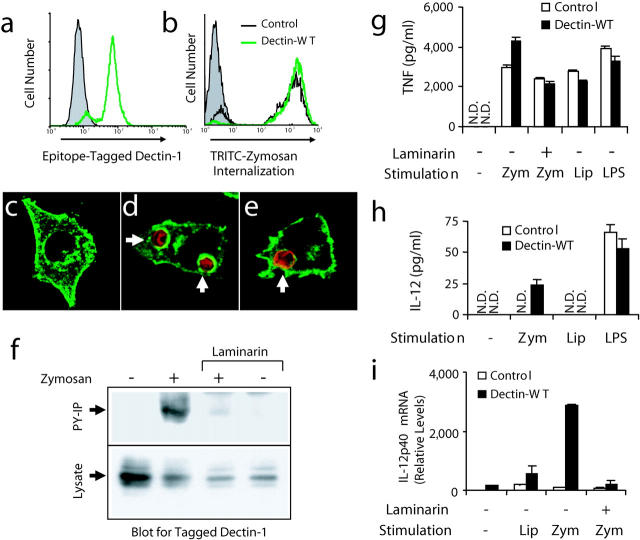
Figure 3.
Zymosan triggers activation of dectin-1 and enhanced inflammatory responses in mouse macrophages. (a) V5 epitope–tagged dectin-1 expression was measured by flow cytometry in RAW cells stably overexpressing wild-type dectin-1 (green line) and in control RAW cells (shaded). (b) Control cells (black line) and cells overexpressing dectin-1 (green line) were incubated with tetramethylrhodamine isothiocyanate zymosan for 1 h and phagocytosis was measured by flow cytometry and compared with unfed control cells (shaded). (c–e) The cellular distribution of epitope-tagged dectin-1 (green) was examined by immunofluorescence microscopy in resting cells (c), cells fed zymosan (red) for 5 min (d), or in cells fed IgG-opsonized sheep red blood cells (red) for 5 min (e). (f) Cells expressing V5-tagged dectin-1 were fed 100 μg/ml zymosan for 15 min in the presence or absence of 50 μg/ml laminarin, total cell lysates were prepared, and the protein was detected in all lysates by immunoblot using an antibody for the tag (bottom). Tyrosine-phosphorylated proteins were immunoprecipitated from the lysates and recovery of dectin-1 was analyzed by immunoblot (top). (g and h) Induction of inflammatory responses was measured by ELISA in control RAW cells and cells overexpressing dectin-1 stimulated with 100 μg/ml zymosan (zym), 100 ng/ml PAM3CSK4 lipopeptide (Lip), or 100 ng/ml LPS for 4 (g, TNF-α) or 24 h (h, IL-12 p40) in the presence or absence of 500 μg/ml laminarin as indicated. N.D., none detected. (i) Induction of IL-12 p40 mRNA was measured by quantitative real-time PCR in cells stimulated for 4 h as in g.