Figure 1.
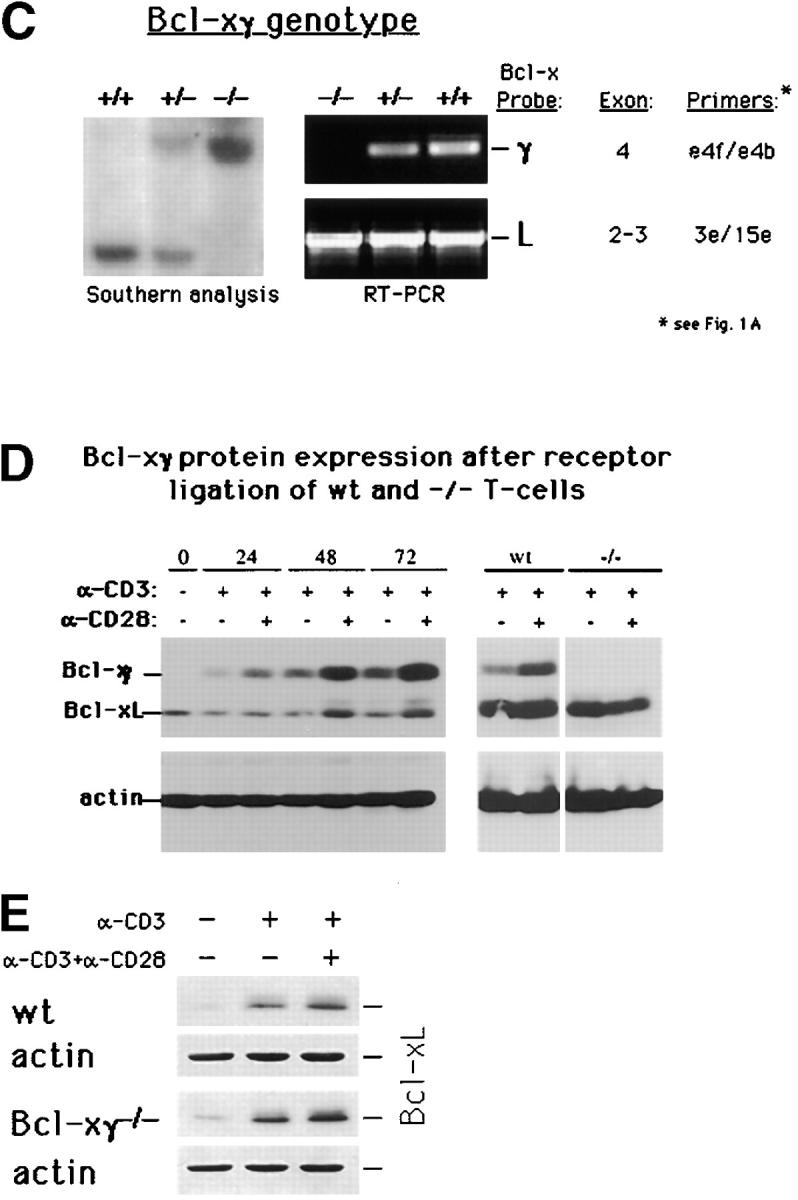
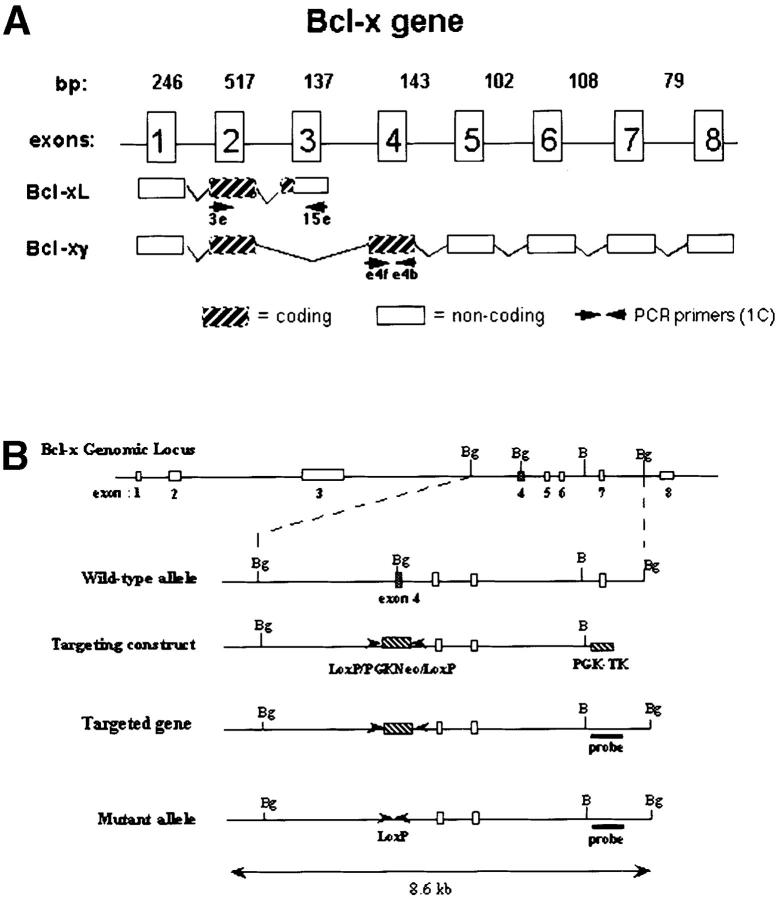
Generation of Bcl-xγ2/− mice. (A) Schematic representation of the coding and noncoding exons of Bcl-xγ and Bcl-xL. Exon 1 encodes the distal portion of the 5′ untranslated region (UTR), and exon 2 encodes the remainder of the 5′ UTR and the common NH2 terminus of Bcl-xL and Bcl-xγ proteins. Exon 3 encodes the COOH terminus of Bcl-xL, as well as the 3′ UTR of Bcl-xL. Exon 4 encodes the unique COOH terminus of Bcl-xγ protein and alternative splicing of exons 5–8 regulates expression of the Bcl-xγ 3′ UTR. (B) Strategy used to generate Bcl-xγ–deficient mice is shown based on targeted disruption of exon 4, the coding region for the unique COOH terminus of Bcl-xγ, as described in Materials and Methods. The genomic structure surrounding exon 4 and the structure of the targeting vector and mutant gene is shown. (C, left) Southern blot of murine Bcl-xγ (+/+, +/−, and −/−) (see Materials and Methods); (right) RT-PCR analysis of RNA (1 μg per sample) derived from Con A-activated LN T cells of Bcl-xγ wt, +/−, and −/− mice. PCR primers used to detect Bcl-xγ and Bcl-xL are indicated in Fig. 1 A and detailed in Materials and Methods. (D) Expression of Bcl-xγ and Bcl-xL protein after CD3/CD28 ligation. (left) Time-course analysis of Bcl-xγ and Bcl-xL protein in T cells from wt mice after CD3 and CD28 ligation. T cells isolated from wt and −/− mice were incubated for the indicated intervals in the presence of plate-bound anti-CD3 antibody (0.2 μg/ml) or anti-CD3 (0.2 μg/ml) and anti-CD28 antibody (1.0 μg/ml); 100 μg of after nuclear supernatant (PNS) loaded per lane. (right) Bcl-xγ and Bcl-xL protein levels in T cells derived from wt and −/− mice activated as described above for 48 h. The upper band (corresponding to Bcl-xγ) was confirmed by reprobing the same blots with an antibody specific to the unique COOH terminus of Bcl-xγ (see Materials and Methods). The immuno-blots shown are representative of one of three independent experiments and were overexposed to provide sensitive detection of trace amounts of Bcl-xγ, while increased levels of Bcl-xL in CD3 and CD3/CD28 cells are noted. Increasing the concentration of plate-bound anti-CD3 alone (up to 5 μg/ml) was not sufficient to induce significant levels of Bcl-xγ in the absence of CD28 ligation. The blots for actin served as a control for protein loading. (E) Expression of Bcl-xL in wt and Bcl-xγ2/− LN after anti-CD3 and anti-CD28 ligation. LN from wt and Bcl-xγ−/− mice were stimulated with plate-bound anti-CD3 or anti-CD3 plus anti-CD28 for 48 h before Bcl-xL expression was confirmed by Western blot analysis with anti–Bcl-x antibody. Expression of Bcl-xL in both strains is similar, indicating that loss of the Bcl-xγ gene does not affect expression of Bcl-xL. Actin was used as a loading control, after membranes were stripped and reprobed with anti-actin antibody.