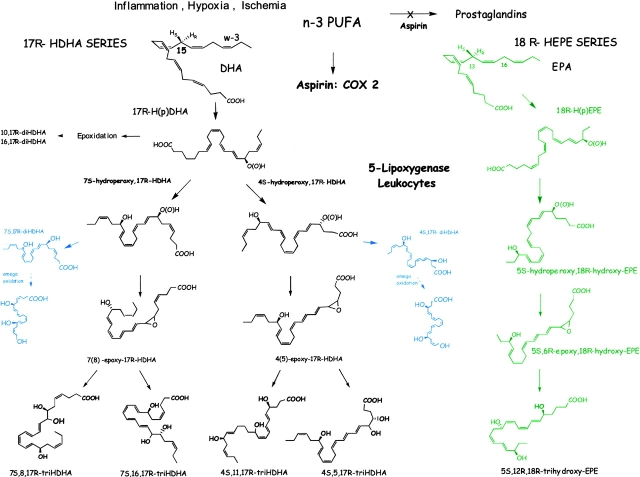
Figure 8.
Biosynthetic scheme proposed for resolvins: aspirin-triggered omega-3-derived products. Acetylation of COX-2 by ASA treatment generates novel 17R-H(p)DHA from DHA that is reduced to its corresponding alcohol and converted via sequential actions of a leukocyte 5–LO and leads to formation of both dihydroxy- and trihydroxy-containing docosanoids that retain their 17R configuration. Pathways are denoted in blue for omega oxidation products that are likely to be in vivo markers of enzymatic inactivation. The resolvin pathways appear to be maximally induced during the “spontaneous resolution” phase of inflammation and compounds are activated to dampen PMN infiltration, which reduces exudate PMN numbers to promote proresolution of inflammatory exudates (resolvins from EPA, the 18R-HEPE series, are denoted in green) that leads to potent inhibitors of PMN recruitment in vitro and in vivo (see pathway, right, text, and reference 2). The complete stereochemistries of the new di- and tri-hydroxy–containing compounds remain to be established and are depicted here in their likely configuration based on biogenic total synthesis. See Table II and text for further details.