Figure 1.
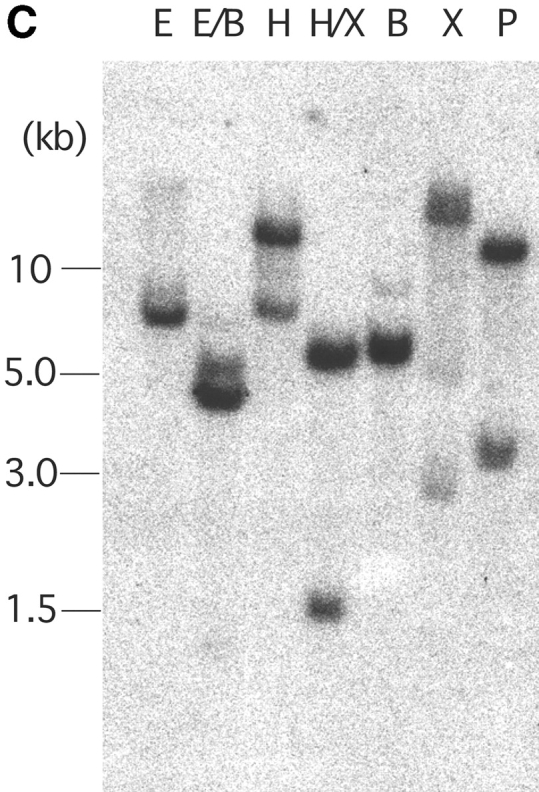
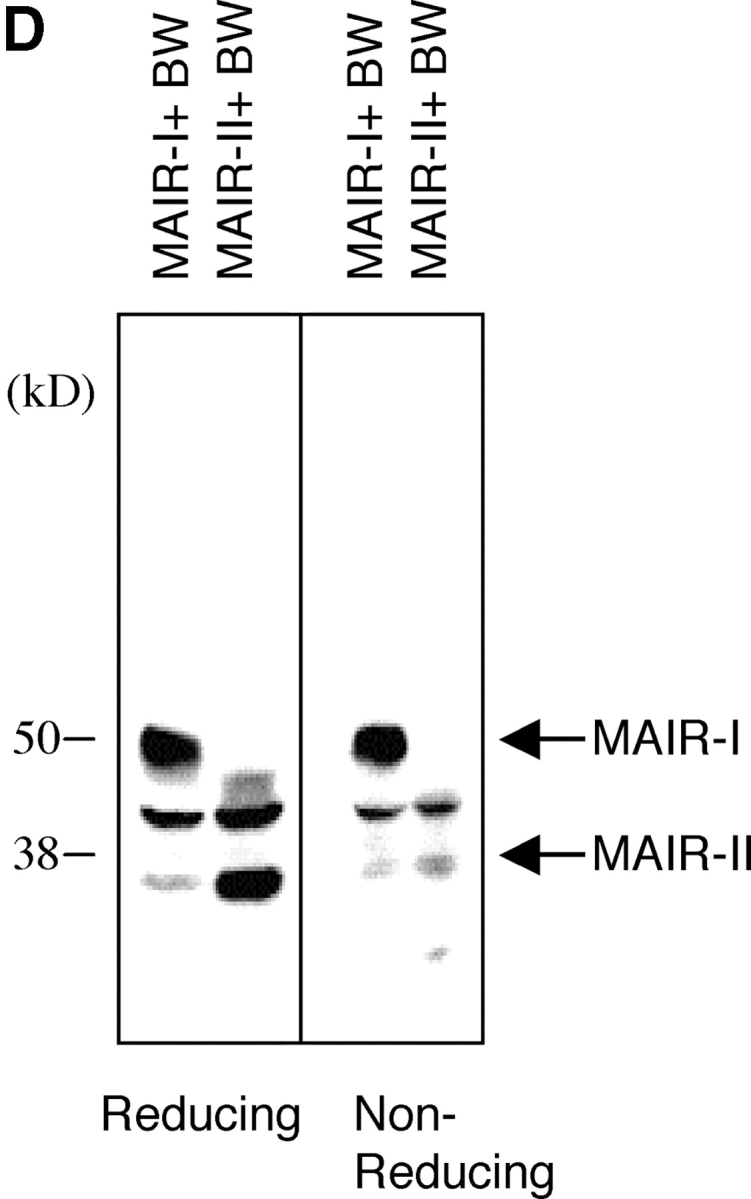
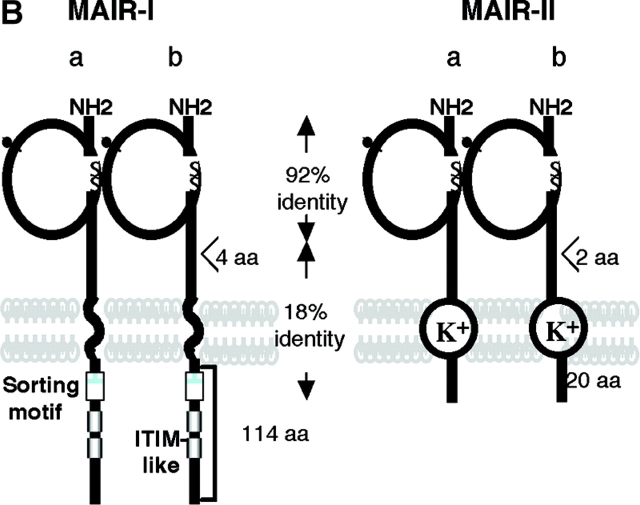
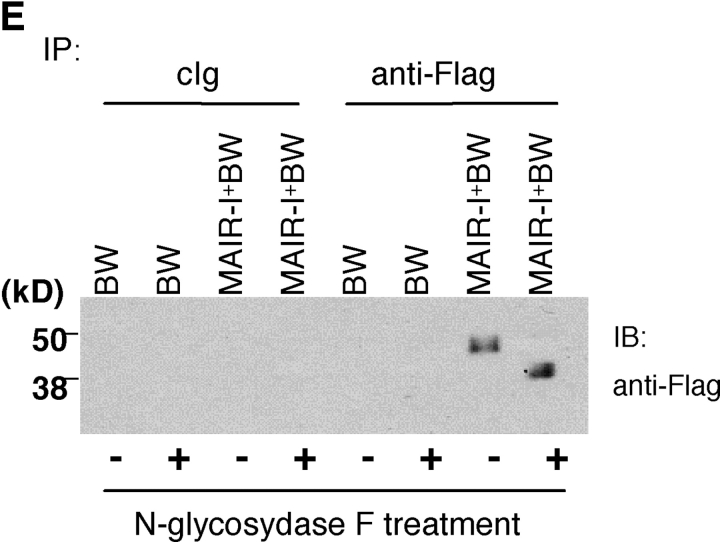
Molecular and biochemical characteristics of MAIR-I and MAIR-II. (A) Predicted aa sequences of MAIR-I and MAIR-II. The putative leader and transmembrane domain are underlined. Potential N-linked glycosylation sites in the extracellular domain and the charged aa residue in the transmembrane region are circled. Potential cysteine residues involved in disulfide bonding of the Ig-like domains are indicated in bold. ITIM-like or sorting motif in the cytoplasmic domain are boxed. The cDNA sequence data are available from GenBank/EMBL//DDBJ under accession nos. AB091765, AB091766, AB091767, and AB091768 (MAIR-Ia, MAIR-Ib, MAIR-IIa, and MAIR-IIb, respectively). (B) Schematic diagram of MAIR-I and MAIR-II proteins. A pair of cysteine residues in the extracellular portion potentially able to participate in intrachain disulfide bonding for the formation of Ig-like domains are indicated. (C) Southern blot analysis of genomic MAIR-I and MAIR-II. Mouse genomic DNA was digested with the indicated enzymes (E, EcoRI; B, BamHI; H, HindIII; X, XbaI; and P, PstI) and probed with the cDNA encoding the extracellular portion of MAIR-I. (D) BW5147 cells expressing the Flag-tagged MAIR-I or MAIR-II were lysed and proteins were analyzed by immunoblotting with anti-Flag mAb under reducing and nonreducing conditions. (E) BW5147 cells expressing the Flag-tagged MAIR-I or parental BW5147 cells were lysed and proteins were immunoprecipitated with anti-Flag. Precipitates were treated or not with N-glycosidase F and immunoblotted with anti-Flag mAb under nonreducing conditions.