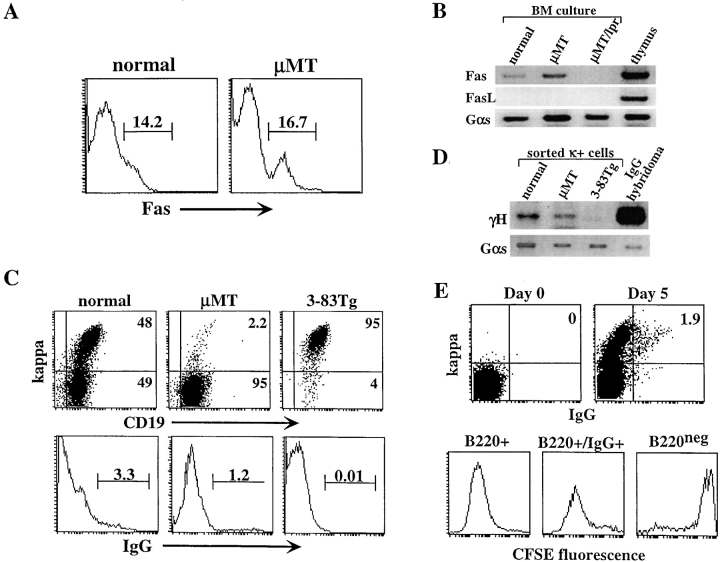
Figure 3.
Lack of FasL allows development of isotype-switched B cell precursors in vitro. BM cells from normal and μMT mice were cultured for 5 d in the presence of IL-7. (A) Cells grown in culture were stained for B220 and Fas receptor and analyzed by FACS®. Expression of Fas was determined in gated B220+ cells. (B) RNA samples from normal μMT and μMT/lpr BM cultures were analyzed by RT-PCR for Fas and FasL gene expression and for Gαs internal control. Thymus RNA sample was used for positive control. In μMT/lpr sample, no product was observed in the PCR for Fas because oligonucleotides used flank the deleted DNA fragment that is found in lpr mice. (C) Surface expression of κ and IgG in BM cultures. BM cells from normal, μMT, and 3-83μδ Tg mice were cultured in vitro, and cells were stained for B220, CD19, κ, and IgG. Analysis for κ (top) and surface IgG expression was performed on 5,000 gated B220+ cells. (D) RT-PCR amplification for detection of γH expression in BM cultures. κ-expressing cells from the indicated BM cultures were sorted using magnetic beads. mRNA samples from the sorted cells were subjected to RT-PCR amplification using oligonucleotides specific for VHJ558 and γCH1. mRNA from IgG-producing hybridoma was used as control. (E) Normal BM cells were depleted of Ig+ cells using magnetic beads, labeled with CFSE on day 0, washed, and cultured for 5 d with IL-7. Cells were stained and analyzed for IgG and κ coexpression on days 0 and 5 (top). Cells grown in the culture were stained for B220 and IgG and analyzed for CFSE fluorescence (bottom). Analysis was performed on gated B220+ cells (left), B220+/IgG+ cells (middle), or B220− cells (right). Results are representative of three different experiments.