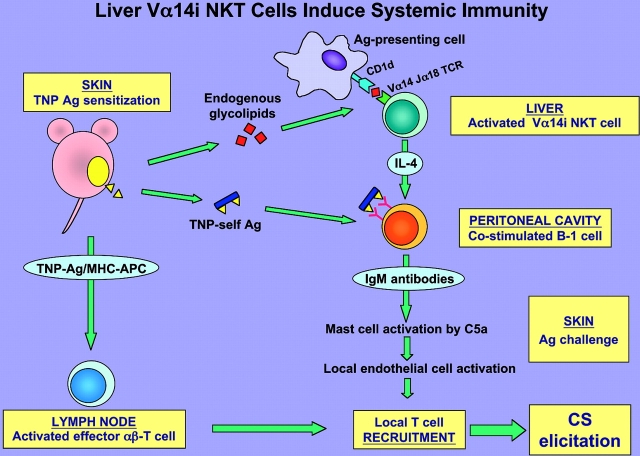
Figure 10.
Summary schema: early activation of liver Vα14i NKT cells leads to initiation of systemic CS immunity. Cutaneous sensitization with PCl (TNP-Cl) is postulated to cause local release from the site of immunization of diverse ligands that act in three directions. First, rapidly after immunization uncharacterized glycolipid ligands stimulate Vα14i NKT cells likely in the liver to produce IL-4. Second, soluble hapten–self-protein and peptide complexes formed by conjugation of the hapten are rapidly released systemically. It is postulated that together the Vα14i NKT cell-derived IL-4 and Ag hapten–self complexes coactivate the B-1 B cells to produce hapten-specific IgM antibodies that circulate within 1 d after sensitization. Third, self-peptides complexed with surface MHC molecules of local APC, such as Langerhans cells, are conjugated with the immunizing hapten to form TNP-peptide–MHC complexes that induce maturation and activation of CS effector memory T cells in the LNs within 4 d after immunization. Subsequently, after secondary ear Ag challenge to elicit CS the local B-1–derived IgM antibodies form complexes with the challenge TNP Ag–self complexes. This activates local complement to generate C5a at the Ag challenge site, leading to release of vasoactive mediators from mast cells that alter the local endothelium, allowing recruitment of the Ag/MHC-restricted CS effector T cells to mediate the late classical 24-h CS response.