Figure 1.
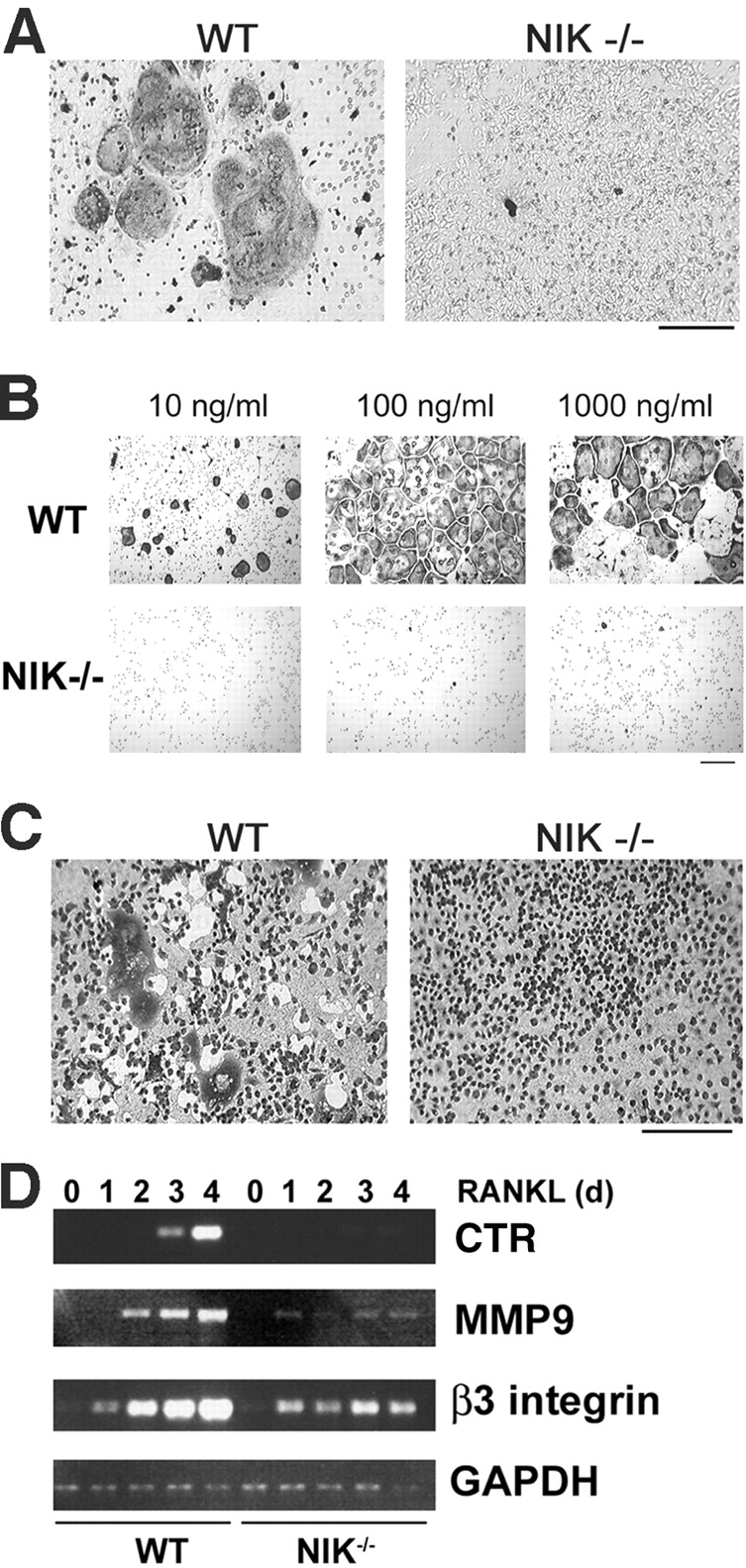
NIK−/− mice have defective RANKL-mediated osteoclastogenesis in vitro. (A) Equal numbers of WT and NIK−/− cells from unfractionated bone marrow were cultured for 8 d in the presence of vitamin D3 and then fixed and stained for TRAP, a marker of OC differentiation. Under these conditions, both stromal cells and OC precursors are present. NIK−/− cultures fail to form large, spread, TRAP-positive OCs. Bar, 300 μm. (B) Equal numbers of Mφs (expanded from bone marrow in high doses of M-CSF) from WT and NIK−/− mice were cultured in the presence of M-CSF and the indicated dose of RANKL for 6 d and then fixed and stained for TRAP. Very few, poorly spread TRAP-positive cells are seen in the NIK−/− cultures. Bar, 100 μm. (C) Osteoclastogenic cultures were generated as in B on an artificial hydroxyapatite matrix (Osteologic slides). On day 6, cultures were stained with methylene blue at basic pH. Resorbed matrix is seen as a clear white area around dark cells. NIK−/− cultures have negligible matrix resorption. Bar, 300 μm. (D) WT and NIK−/− Mφs were treated with RANKL for 0–4 d and then harvested for RNA extraction. Semiquantitative RT-PCR was performed for CTR, MMP9, and β3 integrin, all markers of OC differentiation, with GAPDH as control. Although β3 integrin levels are comparable at day 1, all three markers show a failure in induction from days 2–4 in NIK−/− cultures, confirming the morphological lack of differentiation.
