Figure 2.
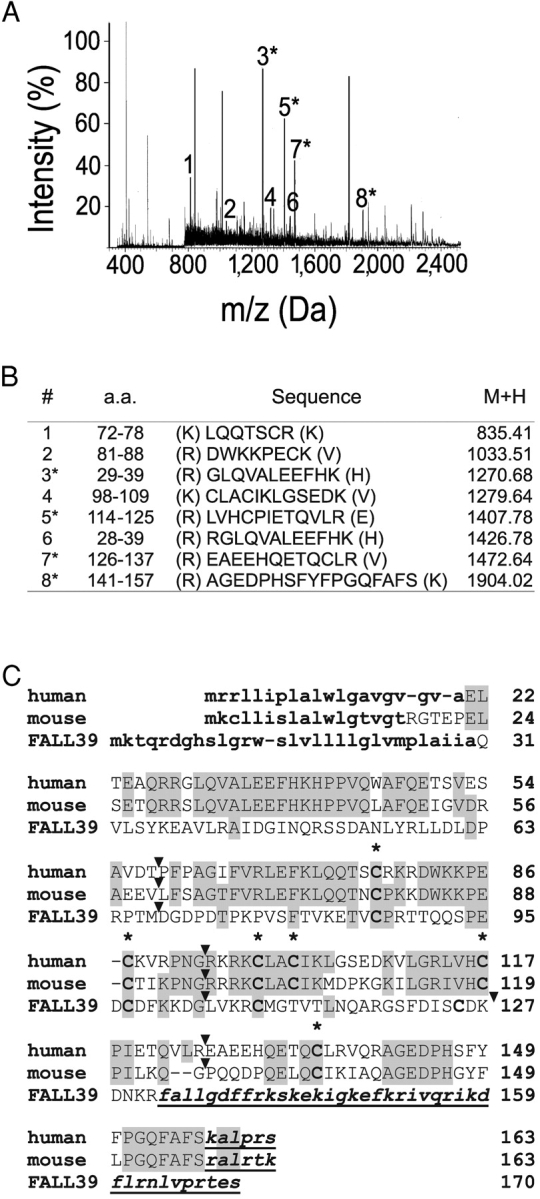
Identification of chemerin as the natural ligand of ChemR23, the chemerin receptor. (A) Monoisotopic peptide mass fingerprinting of the active fraction on a Maldi Q-TOF mass spectrometer after trypsin digestion. (B) Sequences corresponding to selected major peaks of the Maldi Q-TOF mass spectrometer spectrum after trypsin digestion. Peptides 1–7 correspond to tryptic peptides derived from the Tig-2 gene product (prochemerin), whereas peptide 8 is not tryptic and corresponds to the COOH-terminal end of the purified protein. The position of the peptides within this sequence is given. The sequence of peptides in peaks 3, 5, 7, and 8 was confirmed by microsequencing. (C) Amino acid sequence alignment of human and mouse (Protein Data Bank accession no. AK002298) preprochemerin and human cathelicidin FALL39 precursor. Amino acid identities as compared with human preprochemerin are boxed. The signal peptides (predicted for mouse preprochemerin) are in bold lowercase characters and cysteines are in bold. Cleaved COOH-terminal peptides are in bold italics and underlined (predicted by analogy for mouse prochemerin). The location of introns, which interrupt the gene coding sequences between codons, are indicated by arrowheads.
