Abstract
Bile acid transport and secretion in hepatocytes require phosphatidylinositol (PI) 3-kinase-dependent recruitment of ATP-dependent transporters to the bile canalicular membrane and are accompanied by increased canalicular PI 3-kinase activity. We report here that the lipid products of PI 3-kinase also regulate ATP-dependent transport of taurocholate and dinitrophenyl-glutathione directly in canalicular membranes. ATP-dependent transport of taurocholate and dinitrophenyl-glutathione in isolated canalicular vesicles from rat liver was reduced 50–70% by PI 3-kinase inhibitors, wortmannin, and LY294002, at concentrations that are specific for Type I PI 3-kinase. Inhibition was reversed by addition of lipid products of PI 3-kinase (PI 3,4-bisphosphate and, to a lesser extent, PI 3-phosphate and PI 3,4,5-trisphosphate) but not by PI 4,5-bisphosphate. A membrane-permeant synthetic 10-mer peptide that binds polyphosphoinositides and leads to activation of PI 3-kinase in macrophages doubled PI 3-kinase activity in canalicular membrane vesicles and enhanced taurocholate and dinitrophenyl-glutathione transport in canalicular membrane vesicles above maximal ATP-dependent transport. The effect of the peptide was blocked by wortmannin and LY294002. PI 3-kinase activity was also necessary for function of the transporters in vivo. ATP-dependent transport of taurocholate and PI 3-kinase activity were reduced in canalicular membrane vesicles isolated from rat liver that had been perfused with taurocholate and wortmannin. PI 3,4-bisphosphate enhanced ATP-dependent transport of taurocholate in these vesicles above control levels. Our results indicate that PI 3-kinase lipid products are necessary in vivo and in vitro for maximal ATP-dependent transport of bile acid and nonbile acid organic anions across the canalicular membrane. Our results demonstrate regulation of membrane ATP binding cassette transporters by PI 3-kinase lipid products.
The bile canalicular membrane of mammalian hepatocytes contains at least four types of transmembrane proteins that belong to the multidrug resistance or multidrug resistance associated families and that require hydrolysis of ATP for transport of taurocholate [by sister of P-glycoprotein (spgp)], nonbile acid organic anions (by multidrug resistance associated protein 2), organic cations (by multidrug resistance protein 1) and translocation of phosphatidylcholine (PC) from the inner to the outer membrane layer (by multidrug resistance protein 3) (1–5). It recently was demonstrated that taurocholate administration to rats intravenously or by perfusion of isolated rat liver significantly increased biliary secretion and recruitment of each of these transporters from Golgi to the canalicular membrane (6, 7). This process also was associated with an increase in phosphatidylinositol (PI) 3-kinase activity in membrane fractions (7). Administration of wortmannin after perfusion with taurocholate rapidly reduced bile acid secretion <50% of control values but did not affect taurocholate-induced recruitment of the canalicular ATP-dependent transporters (7). These observations suggested that PI 3-kinase lipid products may regulate the activity of spgp in the canalicular membrane.
PI 3-kinase phosphorylates phosphoinositides on the 3′ position of the inositol ring and was initially described in association with receptor and oncogene protein tyrosine kinases (8). Type I PI 3-kinase generates PI 3-phosphate (PI 3-P), PI 3,4-bisphosphate (PI 3,4-P2), and PI 3,4,5-trisphosphate (PI 3,4,5-P3) (9, 10) and requires proximity to its substrates [PI, PI 4-P, and 4,5-bisphosphate (PI 4, 5-P2)] in cellular membranes (11, 12). The catalytic subunit p110 α, β. and δ isoforms are regulated by interaction with the regulatory subunit, p85, whereas p110 γ isoform does not bind p85 and is regulated directly by heterotrimeric G type receptors (13, 14). In addition, the catalytic subunits can be activated by p21ras (15). In recent years, PI 3-kinase activity and its lipid products have been implicated in regulation of many cellular processes, including membrane ruffling, vesicular trafficking, and activation of membrane ion channels (10, 16–19). The precise mechanisms for these effects are not known. Studies of physiological effects of PI 3-kinase products were facilitated by the use of inhibitors, wortmannin, and LY294002. At the concentrations used in our studies, these inhibitors are specific for Type I PI 3-kinase (20–23). Previous studies (7) revealed that taurocholate induces an increase in PI 3-kinase activity and translocation of p85 subunit to the canalicular membrane. The present studies demonstrate that PI 3-kinase activity is necessary for maximal ATP-dependent transport of taurocholate and dinitophenyl-glutathione by rat liver canalicular membrane vesicles and in vivo. Type I PI 3-kinase lipid products directly regulate ATP-dependent membrane transport systems and function of ATP binding cassette membrane proteins.
EXPERIMENTAL PROCEDURES
Materials.
Male Sprague–Dawley rats weighing 250–300 g were purchased from Charles River Breeding Laboratories. Tris⋅Base, EDTA, CaCl2, sucrose, Hepes, taurocholic acid, wortmannin, and all other reagents were purchased from Sigma and were of highest purity. LY294002 was obtained from Calbiochem. The synthetic rhodamine-linked 10-mer peptide that is based on the phosphoinositide-binding sequence of gelsolin (24) was a gift of P. Janmey and J. Hartwig (Brigham and Women’s Hospital, Boston). [3H] taurocholic acid (2–5 Ci/mM), glutathione, [glycine-2-3H] (240 mCi/ mmol), and [γ-32P]ATP (6,000 Ci/mM) were from Dupont/NEN. Dinitrophenyl-glutathione (DNP-GSH) was prepared as described (25).
Perfusion of Isolated Rat Liver.
Rats were anesthetized with sodium pentobarbital (50 mg/kg), and nonrecirculating single pass liver perfusion was performed according to Hems et al. (26). The effect of taurocholate and wortmannin on taurocholate-induced bile acid secretion was determined by liver perfusion at 30 ml/min at 37°C with CO2/O2 (5%/95%) oxygenated Kreb-Ringer’s bicarbonate buffer containing 5.5 mM glucose and 100 μM taurocholate as described (6, 7). [3H] taurocholate (2 × 107 cpm) was added to the buffer after 10 min. Liver viability was assured by maintaining portal pressure (average 10 cm H2O), O2 supply, temperature, and buffer pH (7.35–7.40) throughout the perfusion. Wortmannin 10 mM stock solution was prepared in dimethyl sulfoxide, was diluted to 100 nM in buffer immediately before use, and was infused at 30 ml/min after 30 min of taurocholate perfusion. Bile was collected at 3-min intervals, and samples were weighed to determine volume and taurocholate secretion. The effluent also was collected for measurement of [3H] taurocholate. The liver was removed at the indicated time. Canalicular membrane vesicles were prepared and characterized as described below.
Preparation of Plasma Membrane Vesicles.
Male Sprague–Dawley rats weighing ≈250 g were anesthetized with ether and sodium pentobarbital (50 mg/kg i.p.). The liver was rapidly perfused at room temperature with 0.25 M sucrose and 10 mM Hepes-Tris that contained protease inhibitors (2 μg/ml aprotinin, 2 μg/ml leupeptin, 2 μg/ml pepstatin, 100 μg/ml phenylmethyl-sulfonylchloride, and 5 μg/ml benzamidine) and was homogenized in 5 volumes of buffer. Canalicular membrane vesicles were isolated from liver homogenates by nitrogen cavitation and Ca2+ precipitation (29), and vesicle purity was determined by using leucine aminopeptidase (30) and γ glutamyl transpeptidase (31). With respect to the activity in homogenate, enrichment was 50- to 70-fold with leucine aminopeptidase and 20- to 30-fold with γ glutamyl transpeptidase. The yield of canalicular membrane vesicles was 1–1.2 mg protein/60 g of rat liver. Vesicles were stored in buffer A (10 mM Hepes-Tris, pH 7.4/0.25 M sucrose/0.2 mM CaCl2) at −70°C until used.
Transport Assay.
Transport of taurocholate and DNP-GSH was measured by a rapid filtration method (32). The reaction mixture contained 1.2 mM ATP, ATP regeneration system (3 mM creatine phosphate/100 μg/ml creatine kinase) in buffer B (10 mM Hepes-Tris, pH 7.4/0.25 M sucrose/10 mM MgCl2/0.2 mM CaCl2), and 10 μM taurocholate with [3H] taurocholate or DNP-GSH with [3H] DNP-GSH (1 × 107 cpm/ml). For each time point, transport was initiated by adding 20 μl of reaction mixture that was preincubated for 5 min at 37°C to 20 μl of canalicular membrane vesicles (20–40 μg of protein) suspended in buffer A at 37°C. After the indicated time, the reaction was stopped by addition of 1 ml of ice-cold buffer B. Vesicles were filtered through glass microfiber filters (Whatman; 0.45 μM pore size) and were washed twice with 10 ml of ice-cold buffer B. Radioactivity on the filters was measured by liquid scintillation counter (Beckman Coulter; model LS 1801). A portion (0.2 mM) of PC alone or in combination with 0–20 μM PI 3-P, PI 4,5-P2, PI 3,4-P2, or PI 3,4,5-P3 were sonicated in 10 mM Hepes-Tris (pH 7.4). Vesicles were incubated at room temperature for 5 min with lipids or for 10 min with the synthetic peptide. Wortmannin was incubated with vesicles for indicated times. Transport assays were performed as described above.
PI 3-Kinase Activity.
Assays were performed by using 250 ng of canalicular membrane protein in 0.001% Nonidet P-40, 150 μM ATP, 125 mM Mops (pH 7.0), 25 mM MgCl2, 5 mM EGTA, and 0.2 mg/ml sonicated lipids: phosphatidylserine:PI:PI 4,5-P2 (1:1:1) in sonication buffer (25 mM Mops, pH 7.0/1 mM EGTA) with 25 μCi of [γ-32P] ATP/assay in a total volume of 50 μl (27). Assays proceeded at 37°C for 20 min and were stopped with 100 μl CH3OH:1N HCl (1:1), and lipids were extracted twice with 100 μl of chloroform. The organic layer was combined, was dried under nitrogen, and was analyzed by TLC. [32P]-labeled phosphoinositides were resolved in water:acetic acid:n-propanol (34:1:65) and were detected by autoradiography. [32P] incorporation into PI 3,4,5-P3 was quantified by liquid scintillation counting of TLC spots, which were scraped and eluted in scintillation fluid.
RESULTS
Effect of Wortmannin on Taurocholate-Induced Biliary Secretion in Isolated Perfused Rat Liver.
Intravenous administration of taurocholate at concentrations that approximate the physiological postprandial bile acid load enhances bile acid secretion to ≈4,000 nmol/3 min (6, 7). This process is associated with translocation of all known canalicular ATP-dependent transporters, including the major bile acid transporter, spgp, to the canalicular membrane, activation of canalicular membrane-associated PI 3-kinase, and translocation of its regulatory subunit, p85, to the canalicular membrane (7). Similar effects were achieved by perfusion of isolated rat liver with taurocholate for 30 min (Fig. 1). These observations suggest that taurocholate-induced bile acid secretion is associated with translocation of PI 3-kinase inasmuch as taurocholate has no direct effect on the activity of purified PI 3-kinase (7) or on PI 3-kinase activity in isolated canalicular membrane vesicles (data not shown).
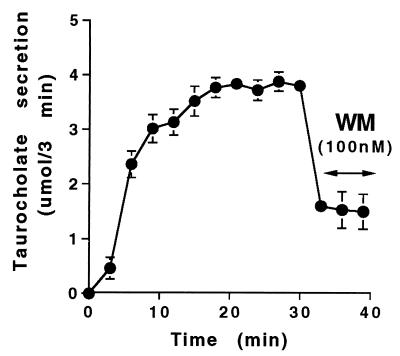
Figure 1.
Effect of wortmannin (WM) on taurocholate-induced bile acid secretion in isolated perfused rat liver. Bile was collected at 3-min intervals in rats receiving taurocholate (100 μM at 30 ml/min for 39 min), which contained 3H taurocholate (1 × 107 cpm/ml). Wortmannin (100 nM) was added to the perfusate for the last 9 min. Results are from one representative experiment, which was reproduced six times. Each point is a mean value obtained from triplicate samples.
Administration of wortmannin for 9 min to taurocholate-perfused isolated rat liver resulted in a 50–70% reduction in bile acid secretion within 3 min (Fig. 1) without affecting taurocholate uptake or taurocholate-induced translocation of spgp and other ATP-dependent transporters to the canalicular membrane (ref. 7; data not shown). The rapid decrease in taurocholate secretion observed by treatment with wortmannin in vivo suggested that wortmannin may have a direct effect on canalicular taurocholate transport and, therefore, that PI 3-kinase may be necessary for bile acid transporter function in the canalicular membrane.
Maximal ATP-Dependent Transport of Taurocholate in the Canalicular Membrane Requires PI 3-Kinase Activity.
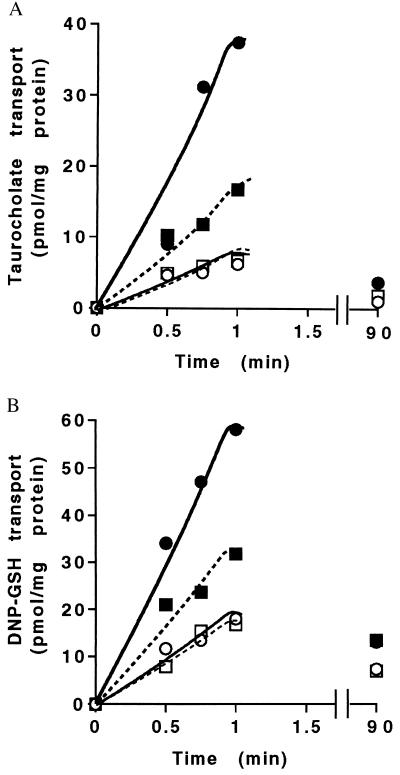
Canalicular membrane vesicles isolated from normal rat liver have proven to be an important system in which to study bile acid secretory mechanisms in vitro (33, 34). Addition of ATP induces a 4- to 6-fold increase in transport of taurocholate and other bile acids in canalicular membrane vesicles whereas minimal basal transport reflects nonspecific binding or diffusion of the radiolabeled material (Fig. 2A). ATP-dependent taurocholate transport was reduced below 50% by addition of 50 nM wortmannin. The inhibitory effect of wortmannin was not limited to taurocholate transport because it also blocked ATP-dependent transport of DNP-GSH, a substrate for multidrug resistance associated protein 2 in the canalicular membrane (Fig. 2B). When maximal ATP-dependent taurocholate transport was measured at 1 min, wortmannin and a different PI 3-kinase inhibitor, LY294002, were inhibitory at IC50 25 nM and 20 μM, respectively. These low concentrations of the inhibitors are specific for Type 1 PI 3-kinase activity (9). Maximal inhibition of ATP-dependent taurocholate transport in canalicular membrane vesicles occurred within 3–5 min after incubation with wortmannin (data not shown), which correlates with the time of decrease in bile acid secretion observed in vivo (Fig. 1) and suggests that ATP-dependent taurocholate transport requires PI 3-kinase products that rapidly recycle in the canalicular membrane.
Figure 2.
Effect of wortmannin and LY294002 on ATP-dependent transport of taurocholate and DNP-GS in canalicular membrane vesicles. (A) Time course of taurocholate transport with or without wortmannin (50nM). ○, −ATP; ●, +ATP; □, −ATP, +wortmannin; ■, +ATP, +wortmannin. (B) Time course of DNP-GS transport with or without wortmannin (50nM). ○, −ATP; ●, +ATP; □, −ATP, +wortmannin; ■, +ATP, +wortmannin.
PI 3-Kinase Products Are Sufficient to Support ATP-Dependent Taurocholate and DNP-GSH Transport in Canalicular Membrane Vesicles.
To determine whether PI 3-kinase products are required for taurocholate and DNP-GSH transport in canalicular membrane vesicles, we examined whether addition of 3′ phosphorylated polyphosphoinositides overcomes the inhibitory effect of wortmannin. As reported, phosphatidylserine but not PC supports PI 3-kinase activity (35). Incubation of canalicular membrane vesicles with PC reduced ATP-dependent taurocholate and DNP-GSH transport almost to background levels whereas phosphatidylserine had no significant effect (Table 1; data not shown). When PI 3-kinase products are presented in micellar form to intact cells, they are incorporated into membranes and elicit cell migration and activation of endogenous enzymes, such as specific protein kinase C isoforms (17, 36). Addition of PI 3,4-P2 to PC-containing micelles resulted in increased taurocholate and DNP-GSH transport, which was resistant to 100 nM wortmannin. The effect of PI 3,4-P2 was dose-dependent with a maximal increase at 10–20 μM (Fig. 3). Wortmannin-inhibitable taurocholate and DNP-GSH transport also were restored by PI 3,4,5-P3 (Table 1) and PI 3-P (data not shown), although less efficiently than by PI 3,4-P2 at similar concentrations. To test specificity of PI 3-kinase lipid products, we also examined the effect of PI 4,5-P2, which did not restore ATP-dependent transport of either taurocholate or DNP-GSH. These results indicate that PI 3-kinase products are sufficient to drive ATP-dependent canalicular transport of taurocholate and DNP-GSH in the absence of active PI 3-kinase.
Table 1.
Effect of phospholipids and WM on ATP-dependent taurocholate and DNP-GSH transport at 1 min in CMV
| Taurocholate transport
|
DNP-GSH transport
|
|||
|---|---|---|---|---|
| CMV | CMV + PC | CMV | CMV + PC | |
| ATP | 32.7 ± 5.9 | 3.5 ± 0.6 | 25.1 ± 2.1 | 4.0 ± 0.8 |
| ATP + WM | 7.5 ± 1.5 | 0.4 ± 0.2 | 8.2 ± 1.1 | ND |
| ATP + PI 3,4-P2 | ND | 49.9 ± 7.5 | ND | 101.5 ± 18.7 |
| ATP + WM + PI 3,4-P2 | ND | 29.8 ± 3.7 | ND | 50.2 ± 10.5 |
| ATP + PI 3,4,5-P3 | ND | 34.6 ± 6.2 | ND | 35.3 ± 4.2 |
| ATP + WM + PI 3,4,5-P3 | ND | 24.2 ± 3.8 | ND | 33.8 ± 4.0 |
| ATP + PI 4,5-P2 | ND | 2.8 ± 0.6 | ND | ND |
Sonicated lipids (200 μM PC or 200 μM PS with 10 μM PI 3,4-P2, PI 3,4,5-P3, or PI 4,5,-P2, final concentrations) were incubated for 10 min with CMV at 37°C. Wortmannin (100 nM) was added for the last 5 min before initiation of transport assay. Transport was measured at 1 min and was expressed as picomoles of taurocholate or DNP-GSH transported/mg protein. The results are expressed as means ± SD for taurocholate (n = 6) and means ± standard error for DNP-GSH (n = 2). ND, not determined; WM, wortmannin; CMV, canalicular membrane vesicle.
Figure 3.
Effect of PI 3,4-P2 on wortmannin-induced inhibition of ATP-dependent transport of taurocholate. Increasing concentrations of PI 3,4-P2 were added to PC-containing micelles. ●, +ATP; ○, +ATP, +wortmannin. Results are presented as means ± SD in two experiments performed in duplicate.
Activation of PI 3-Kinase Induces ATP-Dependent Taurocholate and DNP-GSH Transport.
To determine whether activation of PI 3-kinase in canalicular membrane vesicles enhances taurocholate and DNP-GSH transport, we used a rhodamine-linked synthetic 12-mer peptide that is modeled on the phospholipid-binding sequence of gelsolin and binds several polyphosphoinositides in vitro (24). This membrane-permeable compound elicits accumulation of polyphosphoinositides in macrophages and induces wortmannin-inhibitable cellular responses in platelets and leukocytes (ref. 37; A. Toker and J. Hartwig, personal communication). Incubation of canalicular membrane vesicles for 10 min with synthetic peptide (10 μM) increased PI 3-kinase activity (data not shown) and doubled ATP-dependent canalicular transport of taurocholate and GS-DNP (Fig. 4). The increase in ATP-dependent taurocholate and DNP-GSH transport induced by the synthetic peptide was inhibited by wortmannin at 50 nM (Fig. 4 B and C), which does not inhibit PI 4-kinase (38). ATP-dependent transport of taurocholate by canalicular membrane vesicles obtained from taurocholate-treated rats was increased further by addition of the 10-mer peptide. Similar results were observed when PI 3,4-P2 was added to canalicular membrane vesicles obtained from taurocholate-treated rats (data not shown). These results support the conclusion that PI 3-kinase is necessary for ATP-dependent taurocholate and DNP-GSH transport.
Figure 4.
Effect of synthetic peptide on ATP-dependent transport of taurocholate and DNP-GSH. (A) Effect of peptide concentration on ATP-dependent transport of taurocholate in canalicular membrane vesicles at 1 min. Results are presented as the means of two duplicate samples from a representative experiment. (B) Taurocholate transport at 1 min by canalicular membrane vesicles preincubated with 20 μM peptide for 10 min and 50 nM wortmannin for another 10 min. Results are presented as mean ± SD of three experiments. (C) DNP-GSH transport at 1 min with 20 μM peptide and 50 nM wortmannin as mentioned in B. Results are presented as mean ± SD of three experiments.
Wortmannin Perfusion of Isolated Rat Liver Resulted in Reduced ATP-Dependent Taurocholate Transport in Canalicular Membrane That Was Restored by Addition of PI 3,4-P2.
As shown in Fig. 1, addition of wortmannin to taurocholate-perfused isolated rat liver resulted in rapid reduction in bile acid secretion. We measured PI 3-kinase activity and taurocholate transport in canalicular membrane vesicles isolated from control, taurocholate, or taurocholate- and wortmannin-perfused isolated rat liver. Similar to a previous report (7), canalicular membrane vesicles from liver perfused with taurocholate had a 2-fold increase in PI 3-kinase activity, which was blocked by perfusion with wortmannin for 9 min (Fig. 5A). ATP-dependent transport of taurocholate was enhanced by prior treatment in vivo with taurocholate; however, addition of wortmannin inhibited ATP-dependent taurocholate transport in these canalicular membrane vesicles. Addition of PI 3,4-P2 to the vesicles restored ATP-dependent transport above the level induced by taurocholate (Fig. 5B). These results indicate that wortmannin administration in vivo reduced PI 3-kinase activity and inhibited transfer of taurocholate and DNP-GSH by ATP-dependent transporters in canalicular membrane vesicles. These transport processes were fully restored by addition of PI 3 kinase lipid products to canalicular membrane vesicles.
Figure 5.
PI 3-kinase activity and ATP-dependent taurocholate transport in canalicular membrane vesicles isolated from liver after perfusion with control buffer, taurocholate, or taurocholate and wortmannin. (A) Total PI 3-kinase activity in canalicular membrane vesicles expressed as percent of control values (1.73 ± 0.15 pmol of PI 3,4,5-P3/mg protein/min). Results are means ± SD from three experiments performed in duplicate. (B) ATP-dependent taurocholate transport in canalicular membrane vesicles from liver perfused with buffer (open bars), taurocholate (closed bars), and taurucholate and wortmannin (shaded bars). The same vesicles were preincubated with 10 μM PI 3,4-P2 in PC-containing micelles as indicated. Results are means ± SD from three experiments performed in triplicate.
DISCUSSION
In isolated perfused rat liver, intraportal administration of taurocholate enhanced bile acid secretion, which was inhibited >50% by perfusion with 100 nM wortmannin (Fig. 1; refs. 7 and 39). The maximal effect of wortmannin on bile secretion was evident within 3 min. However, taurocholate-induced translocation of spgp and other ATP-dependent transporters from Golgi to the canalicular membrane was not prevented by subsequent administration of wortmannin (ref. 7; data not shown). Because wortmannin at <100 nM inhibits Type I PI 3-kinase, we propose that 3′ highly phosphorylated polyphosphoinositides may be directly involved in the function of spgp and other canalicular membrane ATP binding cassette proteins. The following evidence supports this hypothesis.
Maximal ATP-dependent transport of taurocholate in canalicular membrane vesicles from normal rat liver was blocked by incubation of vesicles with low concentrations of wortmannin and LY294002 with IC50 of 25 nM and 20 μM, respectively. Inhibition of ATP-dependent taurocholate transport by wortmannin was evident within 3–5 min of incubation with canalicular membrane vesicles, which suggests that PI 3 kinase lipid products undergo rapid turnover in these vesicular fractions. Wortmannin and LY294002 do not directly affect ATP-dependent transporters of taurocholate or DNP-GSH but act through their ability to inhibit PI 3-kinase activity, as evidenced by reversal of their inhibitory effect, specifically by PI 3,4-P2 and less efficiently by PI 3-P and PI 3,4,5-P3 but not by PI 4,5-P2. PC, as opposed to phosphatidylserine, is also inhibitory to purified PI 3-kinase (40). PC inhibited ATP-dependent taurocholate and DNP-GSH transport by canalicular membrane vesicles, which also was reversed by addition of PI 3-kinase lipid products. These data indicate that PI 3-kinase products are necessary for maximal ATP-dependent transport of bile acid and DNP-GSH in canalicular membrane vesicles. Because bile acid secretion and ATP-dependent transport by canalicular membrane vesicles of taurocholate and DNP-GSH was reduced only 50–70% by wortmannin or LY294002, Type I PI 3-kinase lipid products may not be the only factors regulating transporter activity.
To demonstrate that activation of PI 3-kinase enhances transporter activity, we used a synthetic peptide, which activates PI 3-kinase in macrophages (37), lipid-bilayer vesicles (41), and canalicular membrane vesicles. The peptide was twice as effective as ATP alone in enhancing ATP-dependent taurocholate and DNP-GSH transport by canalicular membrane vesicles. The effect of the peptide was inhibited by 50 nM wortmannin, which does not inhibit PI 4-kinases, lending further support to the specific role of PI 3-kinase lipid products in taurocholate and DNP-GSH transport in canalicular membrane vesicles. The importance of PI 3-kinase lipid products in ATP-dependent taurocholate and DNP-GSH transport by canalicular membrane vesicles is further emphasized by the observation that addition of peptide or PI 3,4-P2 enhanced transport in vesicles above that observed after taurocholate administration in vivo.
It has been shown that canalicular membrane vesicles isolated from rat liver perfused with taurocholate had a 2-fold increase in wortmannin-inhibitable PI 3-kinase activity associated with translocation of PI 3-kinase by a microtubular-dependent process to the canalicular membrane, as determined by accumulation of p85 subunit in these membranes (7). These results correlate with the present studies in which canalicular membrane vesicles isolated from taurocholate perfused rat liver manifested 1.5-fold increase in ATP-dependent transport that was inhibited by perfusion with wortmannin. Addition of PI 3,4-P2 enhanced ATP-dependent taurocholate transport above basal levels in these vesicles. Furthermore, incubation of taurocholate with canalicular membrane vesicles or purified PI 3-kinase did not increase PI 3-kinase activity (7). These results indicate that PI 3-kinase activity and its lipid products are necessary for maximal ATP-dependent canalicular transport of taurocholate.
The role of PI 3-kinase lipid products is not restricted to regulation of canalicular ATP-dependent taurocholate transport because the activity of multidrug resistance associated protein 2, which transports non-bile acid organic anions such as DNP-GSH, was similarly blocked by wortmannin and was restored by PI 3,4-P2, PI 3-P, and PI 3,4,5-P3 but not by PI 4,5-P2. The synthetic peptide also increased ATP-dependent transport of DNP-GSH, and the effect was inhibited by wortmannin. Therefore, PI 3-kinase activity is required for the function of both canalicular transporters, spgp, and multidrug resistance associated protein 2. Other ATP binding cassette transporter proteins, including cystic fibrosis transmembrane conductance regulator, may be similarly affected by PI 3-kinase lipid products.
The mechanism whereby PI 3-kinase regulates ATP-dependent transporters is not known; however, a direct interaction with phospholipids has been proposed for multidrug resistance protein 1 protein (42, 43). Recent studies on regulation of the KATP channel by PI 4-P and PI 4,5-P2 (44, 45) suggest that these negatively charged lipids may bind to positive charges in the protein thereby opening the channel. Surface charge does not appear to be involved in regulation of canalicular ATP-dependent transporters. In our experiments, PI 3,4-P2, which has three negative charges, was more effective than was PI 3,4,5-P3, which has four negative charges. In addition, PI 3,4-P2 enhanced ATP-dependent transport whereas similarly charged PI 4,5-P2 was inhibitory. PC, which lacks a negatively charged head group and had no effect on the KATP channel, was inhibitory for ATP-dependent transport probably through inhibition of PI 3-kinase activity (40). These observations suggest that the effect of PI 3-kinase lipid products on ATP-dependent canalicular transport is not mediated by surface charge. Regulation of these transporters by lipids is complex and may involve changes in transporter tertiary conformation, ligand binding, or ATPase activity as has been proposed for P-glycoprotein (46). Alternatively, the lipids may alter accessibility of transporters for their substrates, such as has been demonstrated for PI 3-kinase in lipid-bilayer vesicles (41).
Acknowledgments
Appreciation for advice and reagents is expressed to Alex Toker and Lewis Cantley. This work was supported in part by the NIDDK Digestive Disease Center (Grant DK34928) and Grants DK35652 (to I.M.A.) and CA94536 (to L.V.).
ABBREVIATIONS
- DNP-GSH
dinitrophenyl-glutathione
- spgp
sister of P-glycoprotein
- PI
phosphatidylinositol
- PI 3-P
phosphatidylinositol 3-phosphate
- PI 3,4-P2
phosphatidylinositol 3,4-bisphosphate
- PI 3,4,5-P3
phosphatidylinositol 3,4,5-trisphosphate
- PI 4,5-P2
phosphatidylinositol 4,5-bisphosphate
- PC
phosphatidylcholine
Note Added in Proof
The polyphosphoinositide binding peptide used in this study is further described by Janmey et al. (47).
References
- 1.Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann A F, Meier P J. J Biol Chem. 1998;273:10046–10050. doi: 10.1074/jbc.273.16.10046. [DOI] [PubMed] [Google Scholar]
- 2.Buchler M, Konig J, Brom M, Kartenbeck J, Spring H, Horie T, Keppler D. J Biol Chem. 1996;271:15091–15098. doi: 10.1074/jbc.271.25.15091. [DOI] [PubMed] [Google Scholar]
- 3.Kamimoto Y, Gatmaitan Z, Hsu J, Arias I M. J Biol Chem. 1989;264:11693–11698. [PubMed] [Google Scholar]
- 4.Lincke C R, The I, van Groenigen M, Borst P. J Mol Biol. 1992;228:701–711. doi: 10.1016/0022-2836(92)90855-e. [DOI] [PubMed] [Google Scholar]
- 5.Nies A T, Gatmaitan Z, Arias I M. J Lipid Res. 1996;37:1125–1136. [PubMed] [Google Scholar]
- 6.Gatmaitan Z C, Nies A T, Arias I M. Am J Physiol. 1997;272:G1041–G1049. doi: 10.1152/ajpgi.1997.272.5.G1041. [DOI] [PubMed] [Google Scholar]
- 7.Misra S, Ujhazy P, Gatmaitan Z, Varticovski L, Arias I M. J Biol Chem. 1998;273:26638–26644. doi: 10.1074/jbc.273.41.26638. [DOI] [PubMed] [Google Scholar]
- 8.Cantley L C, Auger K R, Carpenter C, Duckworth B, Graziani A, Kapeller R, Soltoff S. Cell. 1991;64:281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- 9.Fruman D, Meyers R, Cantley L C. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 10.Vanhaesebroeck B, Leevers S J, Panayotou G, Waterfield M D. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- 11.Ling L E, Drucker B J, Cantley L C, Roberts T M. J Virol. 1992;66:1702–1708. doi: 10.1128/jvi.66.3.1702-1708.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varticovski L, Daley G Q, Jackson P, Baltimore D, Cantley L C. Mol Cell Biol. 1991;11:1107–1113. doi: 10.1128/mcb.11.2.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stephens L, Smrcka A, Cooke F T, Jackson T R, Sternweis P C, Hawkins P T. Cell. 1994;77:83–93. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 14.Stoyanov B, Volinia S, Hanck T, Rubio I, Loubtchenkov M, Malek D, Stoyanova S, Vanhaesebroeck B, Dhand R, Nurnberg B, et al. Science. 1995;269:690–693. doi: 10.1126/science.7624799. [DOI] [PubMed] [Google Scholar]
- 15.Rodriquez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Nature (London) 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 16.Toker A, Bachelot C, Chen C-S, Falck J R, Hartwig J H, Cantley L C, Kovacsovics T J. J Biol Chem. 1995;270:1–7. doi: 10.1074/jbc.270.49.29525. [DOI] [PubMed] [Google Scholar]
- 17.Hartwig J H, Kung S, Kovacsovics T, Janmey P A, Cantley L C, Stossel T P, Toker A. J Biol Chem. 1996;271:32986–32993. doi: 10.1074/jbc.271.51.32986. [DOI] [PubMed] [Google Scholar]
- 18.Fan Z, Makielski J C. J Biol Chem. 1997;272:5388–5395. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- 19.Huang C-L, Feng S, Hilgemann D W. Nature (London) 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 20.Arcaro A, Wymann M P. Biochem J. 1993;296:297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlahos C J, Matter W F, Hui K Y, Brown R F. J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 22.Wymann M P, Bulgarelli-Leva G, Zvelebil M J, Pirola L, Vanhaesebroeck B, Waterfield M D, Panayotou G. Mol Cell Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domin J, Pages F, Volina S, Rittenhouse S E, Zvelebil M J. Biochem J. 1997;326:139–147. doi: 10.1042/bj3260139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janmey P A, Lamb J, Allen P G, Matsudaira P T. J Biol Chem. 1992;267:11818–11823. [PubMed] [Google Scholar]
- 25.Awasthi Y C, Garg H S, Dao D D, Partridge C A, Srivastava S K. Blood. 1981;58:733–738. [PubMed] [Google Scholar]
- 26.Hems R, Ross B D, Berry M N, Krebs H A. Biochem J. 1966;101:284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Susa M, Keeler M L, Varticovski L. J Biol Chem. 1992;267:22951–22956. [PubMed] [Google Scholar]
- 28.Varticovski L, Harrison-Findik D, Keeler M L, Susa M. Biochim Biophys Acta. 1994;1226:1–11. doi: 10.1016/0925-4439(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 29.Inoue M, Kinne R, Tran T, Arias I M. J Biol Chem. 1983;258:5183–5188. [PubMed] [Google Scholar]
- 30.Roman L M, Hubbard A L. J Cell Biol. 1983;96:1548–1558. doi: 10.1083/jcb.96.6.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orlowski M, Meister M. Biochem Biophys Acta. 1963;73:679–681. doi: 10.1016/0006-3002(63)90348-2. [DOI] [PubMed] [Google Scholar]
- 32.Nishida T, Gatmaitan Z, Che M, Arias I M. Proc Natl Acad Sci USA. 1991;88:6590–6594. doi: 10.1073/pnas.88.15.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue M, Kinne R, Tran T, Arias I M. J Clin Invest. 1984;73:659–663. doi: 10.1172/JCI111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meier P J, St. Meier-Abt A, Barrett C, Boyer J L. J Biol Chem. 1984;259:10614–10622. [PubMed] [Google Scholar]
- 35.Carpenter C L, Duckworth B C, Auger K R, Cohen B, Schaffhausen B S, Cantley L C. J Biol Chem. 1990;265:19704–19711. [PubMed] [Google Scholar]
- 36.Derman M, A, T, Hartwig J, Spokes K, Falk J, Chen C, Cantley L, Cantley L. J Biol Chem. 1997;272:6465–6470. doi: 10.1074/jbc.272.10.6465. [DOI] [PubMed] [Google Scholar]
- 37.Hartwig J H, Bokoch G M, Carpenter C L, Janmey P A, Taylor L A, Toker A, Stossel T P. Cell. 1995;82:643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 38.Meyers R, Cantley L C. J Biol Chem. 1997;272:4384–4390. doi: 10.1074/jbc.272.7.4384. [DOI] [PubMed] [Google Scholar]
- 39.Folli F, Alvaro D, Gigliozzi A, Bassotti C, Kahn C R, Pontiroli A E, Capocaccia L, Jezequel A M, Benedetti A. Gastroenterology. 1997;113:954–965. doi: 10.1016/s0016-5085(97)70192-6. [DOI] [PubMed] [Google Scholar]
- 40.Carpenter C L, Cantley L C. Biochemistry. 1990;29:11147–11156. doi: 10.1021/bi00503a001. [DOI] [PubMed] [Google Scholar]
- 41.Hubner S, Couvillon A D, Kas J A, Bankaitis V A, Vegners R, Carpenter C L, Janmey P A. Eur J Biochem. 1998;258:846–853. doi: 10.1046/j.1432-1327.1998.2580846.x. [DOI] [PubMed] [Google Scholar]
- 42.Doige C A, Yu X, Sharom F J. Biochim Biophys Acta. 1993;1146:65–72. doi: 10.1016/0005-2736(93)90339-2. [DOI] [PubMed] [Google Scholar]
- 43.Sharom F J. Biochem Soc Trans. 1997;25:1088–1096. doi: 10.1042/bst0251088. [DOI] [PubMed] [Google Scholar]
- 44.Shyng S L, Nichols C G. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- 45.Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker S J, Ruppersberg J P, Fakler B. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- 46.Callaghan R, Berridge G, Ferry D R, Higgins C F. Biochim Biophys Acta. 1997;1328:109–124. doi: 10.1016/s0005-2736(97)00079-5. [DOI] [PubMed] [Google Scholar]
- 47.Jamney, P. A., Cunningham, C. C., Stossel, T. P. & Vegner, R. U.S. Patent 5,846,743.