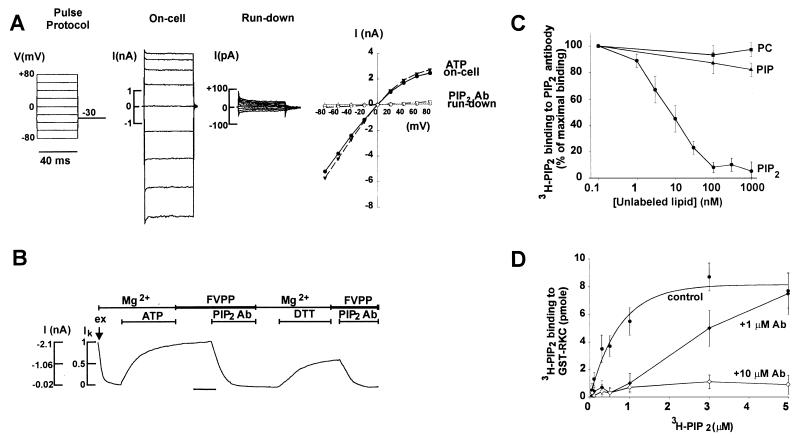
Figure 1.
Inhibition of ATP-dependent channel activation by anti-PIP2 antibody. (A) Pulse protocol and I–V curves for channels on-cell, after run-down, after reactivation by Mg-ATP, and after inhibition by anti-PIP2 antibody as in panel B. The horizontal time bar is 120 sec. (B) Run-down channels were activated by addition of 0.5 mM Mg-ATP (labeled ATP) to the Mg2+ solution. Inward currents (labeled I (nA) in B) was measured and normalized to the on-cell level (IK = 1, for on-cell current; IK = 0, for residual leak current after channels ran down completely). After reaching maximal current, bath solution was changed to FVPP. Anti-PIP2 antibodies (40 nM final concentration in FVPP, labeled PIP2 Ab) inhibited the channels. After washing with Mg2+-containing solution, DTT (2 mM) partially reversed the antibody-induced inhibition. A complete reversal by 2 mM DTT occurred if the membrane patches were washed with FVPP solution (see Fig. 2B), demonstrating that Mg2+ washing activated lipid phosphatases and reduced PIP2 in the membrane. DTT at 0.1–2 mM reversed the inhibition dose-dependently (data not shown). (C) Binding of 3H-PIP2 (10 nM) to anti-PIP2 antibodies (100 nM) in the presence of nonradioactive phosphatidylcholine (PC, ■), phosphatidylinositol 4-phosphate (PIP, ▴]) or PIP2 (●). (D) Binding of 3H-PIP2 to GST–RKC (100 nM) in the absence of antibody (control, ●), in the presence of 1 μM (♦) or 10 μM (⋄) antibody (labeled Ab). Curve fittings were performed assuming one PIP2 binding site for each molecule of GST–RKC or anti-PIP2 antibody. Because 3H-PIP2 is multimeric in the form of liposomes, this analysis may overestimate affinity.