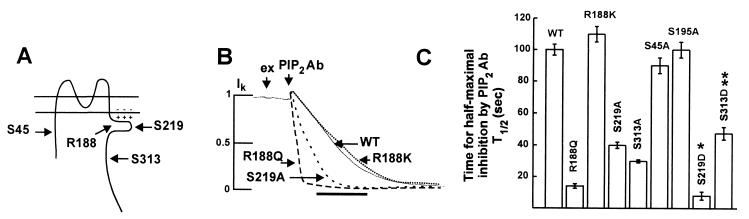
Figure 4.
Serine → alanine mutation at PKA phosphorylation sites reduces channel’s interaction with PIP2. (A) Membrane topology of ROMK1 in relationship to PIP2 in the inner leaflet of the membrane. Like all inward-rectifier K+ channels, it consists of a short N-terminal cytoplasmic domain, two transmembrane domains, one partial membrane domain, and a long C-terminal cytoplasmic tail. The proximal C-terminal region of channel (amino acid 180–223 of ROMK1) contains many conserved basic residues that form the putative PIP2-binding region. A critical role for Arg-188 (R188) in forming an electrostatic interaction with PIP2 is described in the text. Ser-219 (S219) and Ser-313 (S313) are PKA sites that are involved in enhancing PIP2 affinity (see text for details). (B) Membrane patches were excised and stabilized in FVPP solution. Anti-PIP2 antibodies (40 nM in FVPP) were applied to inhibit the channels. The on-cell currents for wild-type (WT) and mutant channels were normalized and superimposed. Time bar is 120 sec. (C) Half-time (t1/2) for maximal inhibition by anti-PIP2 antibody (40 nM) for the wild-type (WT) and the mutant channels of ROMK1. Mean ± SEM, n = 4–16 for each group. ∗ indicates P < 0.01 by unpaired t test, S219D vs. S219A. ∗∗ indicates statistically insignificant, S313D vs. S313A.