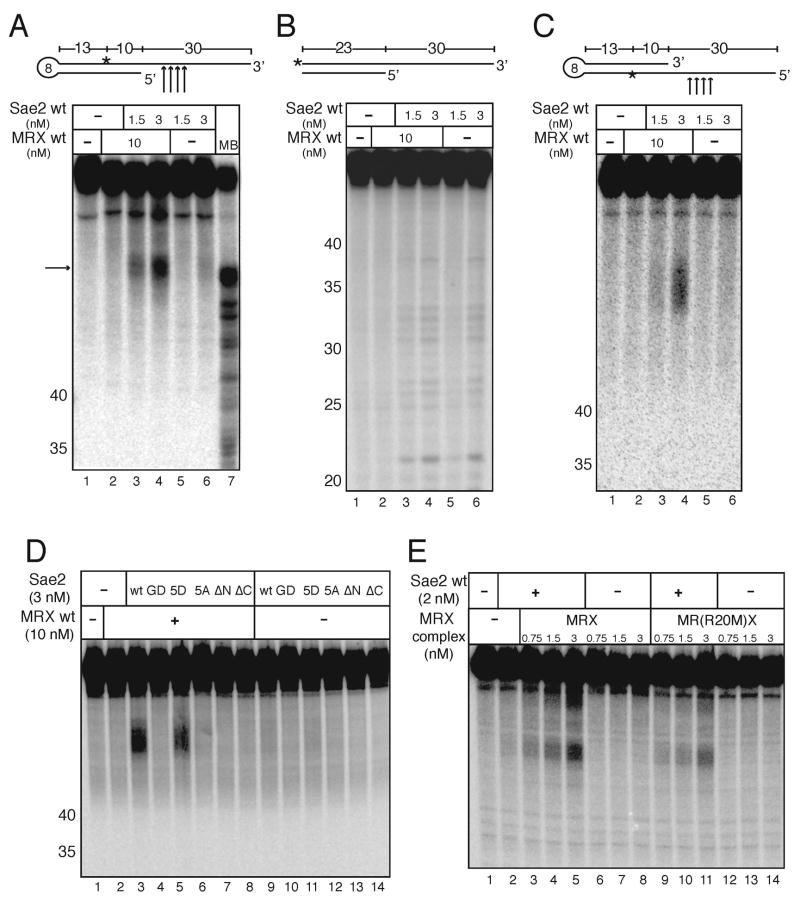
Figure 4.
Sae2 and MRX cooperatively cleave hairpin structures. (A) Wild-type MRX and Sae2 were incubated in 5 mM MgCl2 with an internally [32P]-labeled hairpin DNA substrate as in Fig. 3B. The primary cleavage products (arrow) are not cut at the tip of the hairpin but in the single-stranded overhang as shown in the diagram. MB control as in Fig. 3. (B) Reactions were performed as in (A) but with substrates lacking the hairpin loop. (C) Wild-type MRX and Sae2 were incubated in 5 mM MgCl2 with an internally [32P]-labeled hairpin DNA substrate with the opposite polarity compared to the substrate shown in (A). (D) Wild-type MRX and wild-type and mutant Sae2 proteins were incubated in 5 mM MgCl2 with an internally [32P]-labeled hairpin DNA substrate as in (B). (E) Wild-type Sae2 and wild-type MRX and Rad50S MR(R20M)X complexes were assayed as in (A).