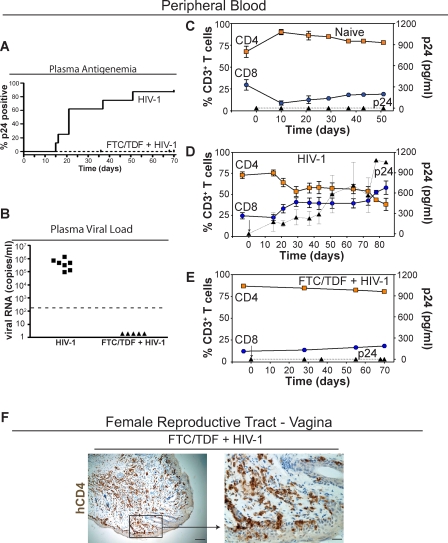
Figure 2. Pre-exposure Prophylaxis Prevents Intravaginal HIV-1 Transmission in Humanized BLT Mice.
(A) Kaplan-Meier plot of the time course to plasma antigenemia conversion following intravaginal HIV-1 exposure in BLT mice with or without the 7-d pre-exposure regimen of FTC/TDF (administered once daily starting 48 h prior to intravaginal inoculation).
(B) Plasma from the seven infected BLT mice and the five FTC/TDF + HIV-1 mice was tested for the presence of HIV-1 RNA. Data presented depict the initial positive viral RNA value for each mouse examined. The dashed line indicates the limit of detection for this assay.
(C–E) Shown are the levels of human CD4+ (orange squares) and CD8+ (blue circles) T cells in peripheral blood as well as the levels of HIV p24 antigenemia (black triangles) in plasma for (C) naive control (n = 6), (D) HIV-1 infected (n = 7), and (E) pre-exposure FTC/TDF-treated animals (n = 5). In (D), note that in seven of eight tested BLT mice, a single exposure to HIV-1 led to intravaginal transmission and an initial drop, with subsequent stabilization, in the levels of peripheral blood CD4+ human T cells. In contrast, no changes were observed in either the naive control (C) or BLT mice that received FTC/TDF for pre-exposure prophylaxis (E). In (D) and (E), day 0 is the day of inoculation and is indicated by an arrow. Gating strategy for flow cytometric analysis: live cells → human CD45 → human CD3 → human CD4 or CD8.
(F) Immunohistochemical staining for human CD4+ cells within the vagina of a representative FTC/TDF-treated mouse demonstrating the continued presence of CD4+ T cells in this tissue (left, bar indicates 50 μm; right, bar indicates 12.5 μm; box indicates region magnified in subsequent image).