Figure 3.
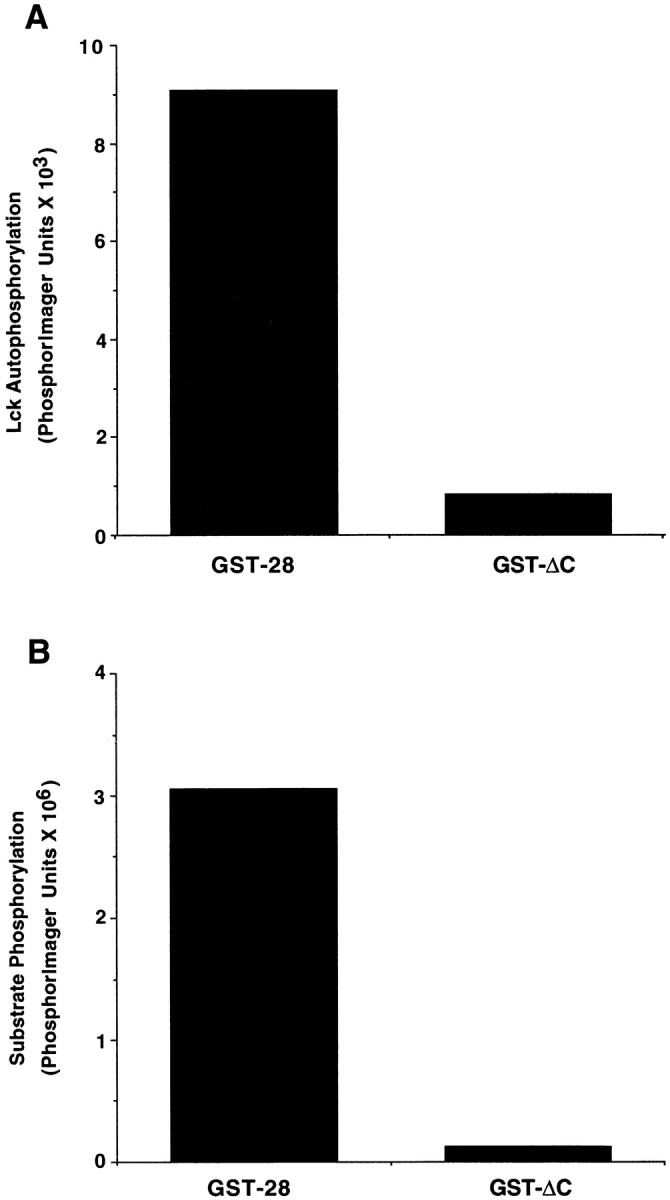
Lck interacts with the last 16 residues in the CD28 tail. (A) Chimeric GST molecules containing the full length CD28 tail or lacking the last 16 residues of CD28 (ΔC) were incubated with Sf9 cell lysates with or without exogenously expressed Lck. GST fusion proteins were isolated with glutathione agarose beads and washed three times. In vitro kinase assays were performed in the presence of [γ-32P]ATP. Kinase reactions were analyzed by SDS-PAGE, and Lck autophosphorylation was quantitated by PhosphorImage analysis. This data is representative of three independent trials. (B) Procedure was the same as described in A, except that in vitro kinase assays were performed in the presence of a specific tyrosine kinase substrate peptide. Kinase reactions were incubated with 10% TCA to precipitate phosphorylated proteins. Peptide phosphorylation was quantitated after binding to phosphocellulose paper using a PhosphorImager. Background kinase activity, determined by measuring activity in reactions prepared with GST alone, was subtracted from all samples. The data is representative of three independent trials.
