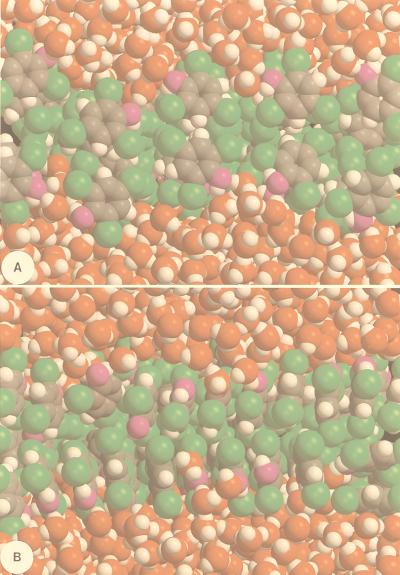
Figure 4.
Front (A) and side (B) views of a 2,4,6-trichlorophenol/water bilayer system, in the directions Oy and Ox parallel to the interface, respectively, obtained from an HMC simulation in the (N, P⊥, γ, T) ensemble. The normal pressure to the interface, P⊥, the surface tension, γ, and the temperature, T, were set at 1 atm, the experimental value of 48.4 mN/m, and 297.1 K, respectively. At thermodynamic equilibrium, the molecular area of 2,4,6-trichlorophenol in the x, y-plane was ca. 36.5 Å2. Each HMC pass corresponded to 250 molecular dynamics steps, by using a 1.5-fs time-step. The system was equilibrated for 10,500 HMC steps. The parameters of the effective potential energy function were taken from the Amber all-atom force field (31), except for the chlorine atoms, the van der Waals parameters of which were recalibrated from state-of-the-art quantum chemical dimerization energies and experimental free energies of hydration—R*Cl = 1.953 Å and ɛCl = 0.350 kcal/mol. Nonbonded interactions between small, electrically neutral groups of the chlorophenols and water were smoothly truncated beyond 9.0 Å. Color code: carbon, gray; 2,4,6-trichlorophenol oxygen, magenta; water oxygen, red; chlorine, green; and hydrogen, white.