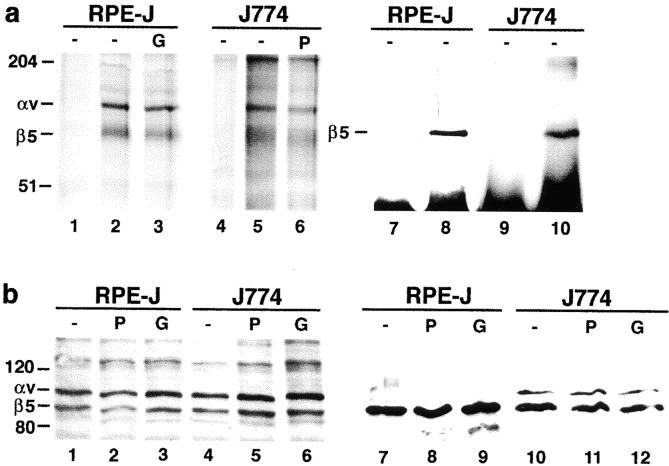
Figure 8.
PKC activation or inhibition does not alter levels or plasma membrane localization of αvβ5 receptors. RPE-J cells were pretreated with solvent (−) or PMA for 30 min (P) or with Gö6976 for 3 h (G) before lysis. J774 cells were pretreated with solvent (−), PMA (P), or Gö6976 for 30 min (G), and lysed. (a) αvβ5 heterodimer levels. Immunoprecipitates from total IP lysate with nonimmune mouse IgG (lanes 1, 4, 7, and 9), or with αvβ5 antibody P1F6 (other lanes). Lanes 1–6 are fluorographs after immunoprecipitation from radioactively labeled cells and nonreducing SDS-PAGE. Lanes 7–10 are immunoblots after immunoprecipitation from unlabeled RPE-J and J774 lysates detected with β5 antibody B5-IVF2 after reducing SDS-PAGE. P1F6 antibody precipitated αvβ5 heterodimers from both metabolically labeled RPE-J and J774 cells, and quantification revealed ∼25% higher levels in the RPE (compare lane 2 with lane 5; relative intensities RPE 1.0/J774 0.78). Immunoblotting of β5 with antibody P1F6 gave similar quantification results and further confirmed the specificity of P1F6 for rodent αvβ5. Pharmacological activation or inhibition of PKC did not influence αvβ5 receptor levels in either cell type, and intensity differences were not significant. (b) Cell surface localization of αvβ5. Cells were pretreated and surface biotinylated before lysis. In lanes 1–6, immune complexes precipitated with P1F6 from biotinylated lysates were probed with streptavidin-peroxidase/ECL after reducing SDS-PAGE and blotting. Similar steady state levels of surface αvβ5 receptors were detected in RPE-J or J774, regardless of PKC inhibition or activation. Note that, under reducing conditions, the αv subunit appears at a lower molecular mass than under nonreducing conditions (∼110 kD reduced, ∼140 kD nonreduced). Other bands, possibly coprecipitates, were not identified. Lanes 7–12 show surface protein fractions enriched by streptavidin-agarose precipitation separated by reducing SDS-PAGE. Immunodetection with β5 antibody B5-IVF2 confirmed equal amounts of β5 protein at plasma membranes of RPE-J cells, regardless of PKC status (lanes 7–9). The same was true for J774 cells (lanes 10–12). One of three representative experiments is shown for each method. In both types of experiments, minor variations did not correspond to PKC modulation and were insignificant.