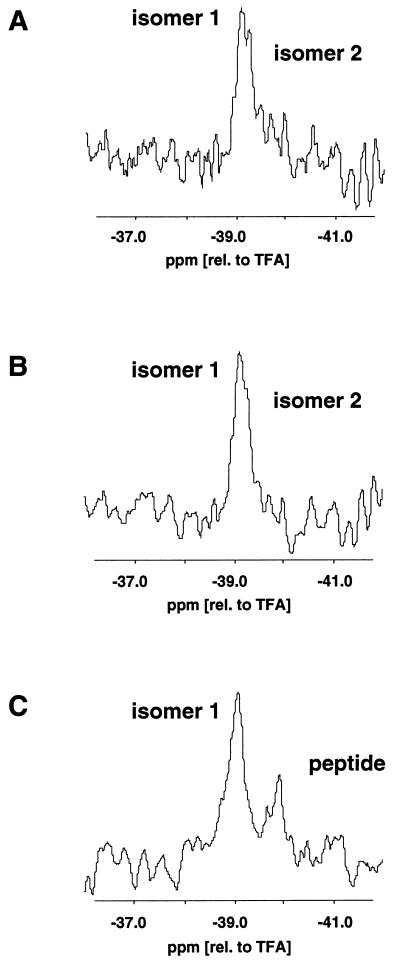
Figure 5.
19F NMR spectra of 0.08 mM F-PCC-(89–104) A96A-19F, Y97Fp19F, A103A-19F/I-Ek in the presence of various concentrations of dynA-(1–13). Shown are only the spectra of Y97Fp19F bound to the P3 pocket of I-Ek. (TFA, trifluoroacetic acid). (A) 0.8 μM dynA (molar ratio of MHC-peptide complex to dynA = 100:1). (B) 0.1 mM dynA (molar ratio of MHC-peptide complex to dynA = 1:1.25). (C) 0.8 mM dynA (molar ratio of MHC-peptide complex to dynA = 1:100). Based on a binding constant Kb of 15 μM−1 of dynA to the MHC-peptide complex a sigmoidal binding curve was calculated for a complex concentration of 0.08 mM. The concentrations of dynA in the 19F NMR experiments correspond to the lower asymptotic part of the sigmoidal binding curve (A), near the 50% value (B), and the upper asymptotic part of the sigmoidal binding curve (C). In A, two 19F NMR signals were observed for Y97Fp19F (labeled isomer 1 and 2). In B, the signal of isomer 2 is reduced to a shoulder. In C, only the signal of isomers 1 was detected in addition to the signal of unbound peptide. In all three spectra, the chemical shift of isomer 1 was identical within experimental error (±0.05 ppm).