Figure 2.
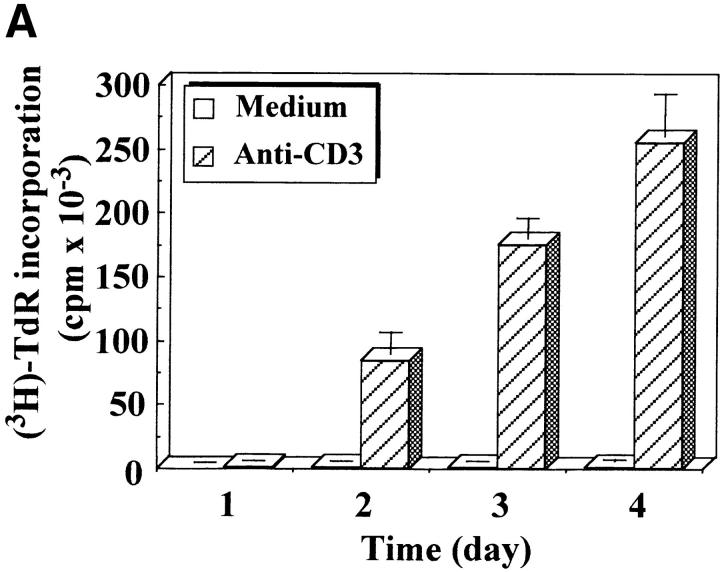
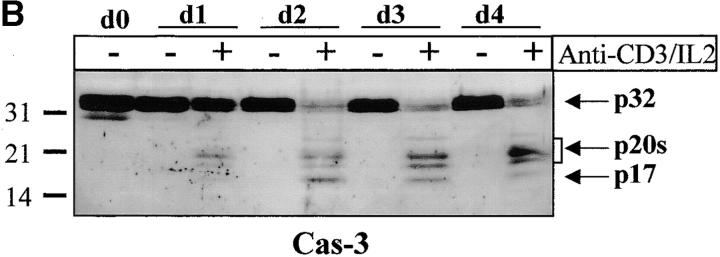
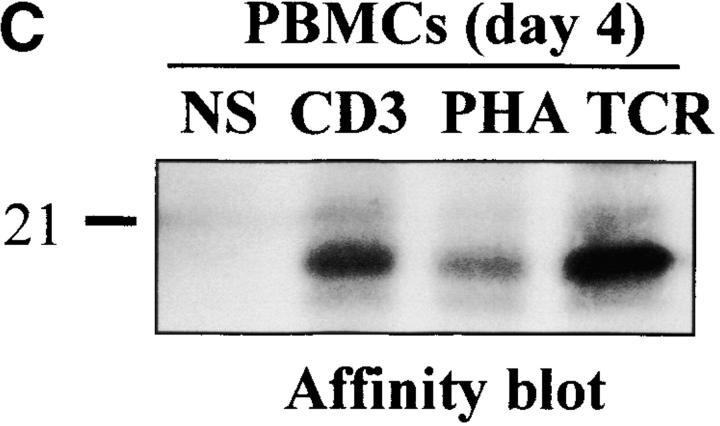
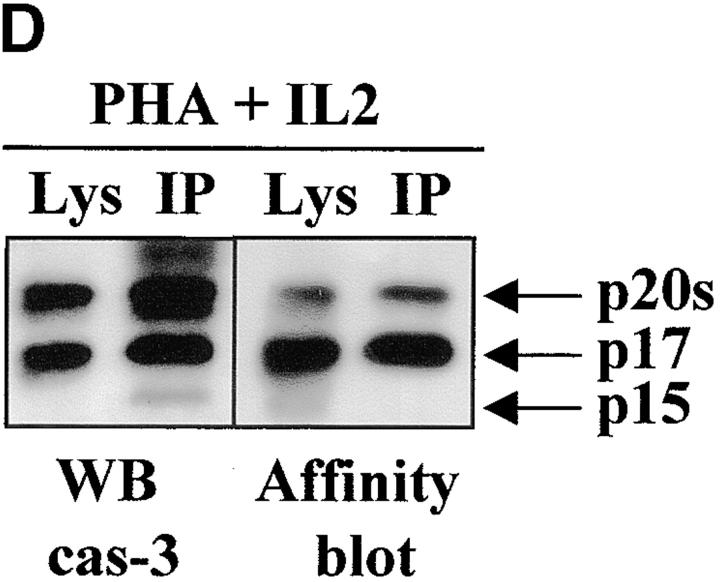
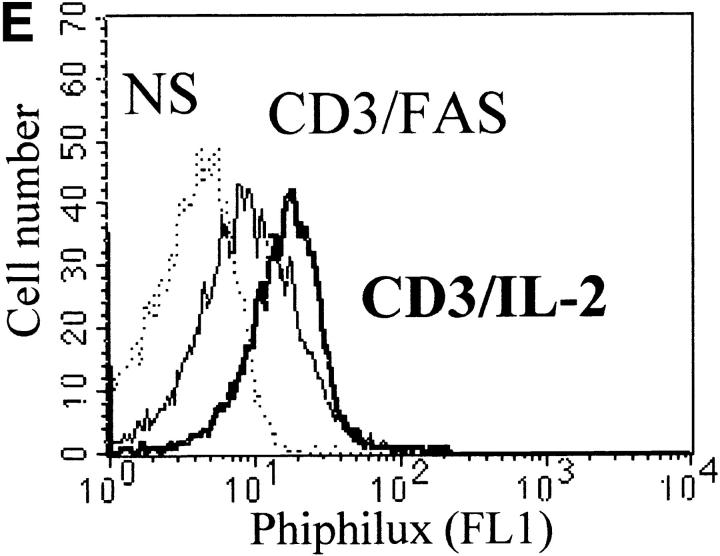
Caspase-3 is cleaved into multiple fragments in stimulated lymphocytes. (A) Proliferation. Purified PBMCs were obtained through Ficoll gradient separation and stimulated with 1 μg/ml of anti-CD3 and 20 U/ml of IL-2 (Anti-CD3) or not (Medium). After 1–4 d at 37°C, thymidine incorporation was determined as in the legend to Fig. 1. (B) Caspase-3 Western blot. Proteins were extracted from the same PBMCs treated in A by cell lysis using a denaturing Laemmli buffer containing 8 M urea. Total proteins extracted from 106 fresh (d0), unstimulated (−), or stimulated cells (+) were separated on SDS-PAGE, and Western blot analysis was performed using a polyclonal rabbit anti–caspase-3 (reference 5). (C) Detection of active caspases by affinity blot. PBMCs stimulated or not for 4 d with anti-CD3, anti-TCR, or PHA in the presence of IL-2 were lysed using a nondenaturing lysis buffer (see Materials and Methods), and lysates were incubated with 5 μM of a biotinylated zVAD peptide, followed by a separation by SDS-PAGE and Western blot analysis using Extra-HRP. (D) Identification of active subunits of caspase-3. PBMCs were activated for 4 d with PHA and IL-2, and cell lysates were incubated with the substrate. Caspase-3 immunoprecipitation was then performed on half of the lysate. Total extract (Lys) or immunoprecipitated caspase-3 (IP) was subjected to Western blot analysis using the anti–caspase-3 antibody (left, WB cas-3) or with Extra-HRP (right, Affinity blot). Results are from one of at least three independent experiments giving similar results. (E) Detection of caspase activity in intact cells. Fresh PBMCs were left untreated (NS) or stimulated (CD3/IL-2) with anti-CD3 antibody and IL-2 for 4 d. A fraction of these activated cells was then incubated for 6 h in anti-Fas–coated plates (CD3/Fas). After a 1-h labeling with the cell-permeable substrate Phiphilux, the cells were analyzed by flow cytometry. Results are from one representative experiment out of three.