Abstract
The administration of concanavalin A (Con A) induces a rapid severe injury of hepatocytes in mice. Although the Con A–induced hepatitis is considered to be an experimental model of human autoimmune hepatitis, the precise cellular and molecular mechanisms that induce hepatocyte injury remain unclear. Here, we demonstrate that Vα14 NKT cells are required and sufficient for induction of this hepatitis. Moreover, interleukin (IL)-4 produced by Con A–activated Vα14 NKT cells is found to play a crucial role in disease development by augmenting the cytotoxic activity of Vα14 NKT cells in an autocrine fashion. Indeed, short-term treatment with IL-4 induces an increase in the expression of granzyme B and Fas ligand (L) in Vα14 NKT cells. Moreover, Vα14 NKT cells from either perforin knock-out mice or FasL-mutant gld/gld mice fail to induce hepatitis, and hence perforin–granzyme B and FasL appear to be effector molecules in Con A–induced Vα14 NKT cell–mediated hepatocyte injury.
Keywords: IL-4, Vα14 NKT cell, Con A, hepatitis, autocrine
Introduction
The Vα14 NKT cells are characterized by coexpression of NK1.1, an NK cell marker, and an invariant antigen receptor encoded by Vα14Jα281 gene segments associated with highly skewed sets of Vβs, mainly Vβ8.2 (Vα14 NKT cell receptor; references 1 2 3 4 5 6 7). Three lines of evidence suggest that Vα14 NKT cells are a novel lineage of lymphocytes: (a) a targeted disruption of the invariant Vα14Jα281 gene causes a selective loss of Vα14 NKT cells, leaving other lymphocytes intact 8; (b) the transgenic expression of the invariant NKT antigen receptor (Vα14/Vβ8.2) genes in recombination activating gene (RAG)1 knock-out (KO) mice results in the exclusive development of Vα14 NKT cells but not other lymphocytes, including T cells 9; and (c) Vα14 NKT cells develop at an early stage of embryogenesis before thymus formation 10.
It has been reported that Vα14 NKT cells specifically recognize α-galactosylceramide (α-GalCer; references 9 and 11) or parasite (malaria or trypanosoma) glycosylphosphatidylinositols 12, both of which are presented by a monomorphic class Ib molecule, CD1d. Upon activation with α-GalCer, Vα14 NKT cells exert a perforin-dependent antitumor cytotoxicity and prevent tumor metastasis in the liver or lung 11. Interestingly, the activated Vα14 NKT cells produce both IL-4 and IFN-γ 9 13 14. Thus, it is important to know whether Vα14 NKT cells behave like either Th1 or Th2 cells, whose dysfunction or imbalance may affect immune responses and lead to disease development. In fact, IFN-γ but not IL-4 produced by activated Vα14 NKT cells produces dominant functional effects on helper T cell differentiation by suppressing Th2 development and IgE antibody responses 15. We and others have demonstrated that a selective loss or dysfunction in Vα14 NKT cells is tightly associated with autoimmune disease development in lpr/lpr and nonobese diabetic mice 16 17 18. In fact, the transfer of the invariant Vα14 gene into nonobese diabetic mice prevents disease development 19. In addition, Vα14 NKT cells appear to play a critical role in liver injury after Salmonella infection 20. Similarly, human NKT cells have been reported to play significant roles in various human autoimmune diseases, including autoimmune diabetes 21 and systemic sclerosis 22. These results suggest that Vα14 NKT cells play a crucial role in various immune responses, including antitumor immunity, allergic reaction, and the development of autoimmune diseases.
Con A–induced hepatitis is considered to be an experimental murine model of human autoimmune hepatitis 23. The hepatocyte injury is associated with lymphocyte infiltration, suggesting the involvement of immune reactions. In fact, this hepatitis is not observed in athymic nude mice or SCID mice 23, both of which lack T cells. Furthermore, in vivo treatment with anti–Thy-1 or anti-CD4 mAbs or with FK506 or cyclosporin A prevents the hepatitis, suggesting a requirement for CD4+ T cells or TCR-mediated signals for Con A–induced hepatitis 23. In addition, immunohistological analyses confirm the infiltration of CD4+ T cells in the portal area of the liver 24.
Recently, Toyabe et al. reported the involvement of NK1.1+ T cells in Con A–induced hepatitis 25. When NK1.1+ cells are depleted by the administration of anti-NK1.1 antibody in vivo, the mice become resistant to the hepatitis, suggesting that NK1.1+ cells are indispensable 25. However, hepatitis does not develop in β2 microglobulin KO mice, in which the development of Vα14 NKT cells is severely perturbed, despite the normal development of NK cells 25. Thus, it is conceivable that Vα14 NKT cells rather than NK cells are involved in Con A–induced hepatitis, although no direct experiments addressing this issue have been reported.
The hepatic injury seems to be induced by several different mechanisms, such as those involving the Fas–Fas ligand (L) system 26 27 28, perforin–granzyme system 29, and IFN-γ– 26 30 and/or TNF-α–mediated cytotoxicity 24 25 31 32 33 34. However, the results are controversial, and the precise molecular requirements for the induction of hepatocyte injury by Con A are still largely unknown. In this report, we investigate the role of Vα14 NKT cells in Con A–induced hepatitis using two strains of gene-manipulated mice established in our laboratory: (a) Vα14 NKT KO mice, where only the Vα14 NKT cells are deficient and other lymphoid populations, such as T cells, B cells, and NK cells, remain intact 8; and (b) Vα14 NKT (Vα14 transgenic (Tg)/Vβ8Tg/RAG KO) mice that have only Vα14 NKT cells and not T cells, B cells, or NK cells 9. Our results suggest that Vα14 NKT cells are required and sufficient for the development of Con A–induced hepatitis. In addition, IL-4 produced by Con A–activated Vα14 NKT cells appears to upregulate FasL and granzyme B expression in Vα14 NKT cells in an autocrine fashion and is indispensable for the induction of the hepatitis.
Materials and Methods
Mice.
8–10-wk-old male C57BL/6 (B6) mice and B6-gld/gld mice were purchased from Japan SLC Inc. Vα14 NKT KO mice were established by specific deletion of the Jα281 gene segment with homologous recombination and aggregation chimera techniques 8 and were backcrossed nine times with B6 mice. Vα14 NKT (Vα14Tg/Vβ8.2Tg/RAG KO) mice were established and backcrossed four times with B6 mice 9. IL-4 KO mice were originally generated by Kopf et al. 35. IFN-γ KO 26 and perforin KO mice 36 were provided by Dr. Y. Iwakura (Institute of Medical Science, University of Tokyo, Tokyo, Japan). RAG KO mice were provided by Dr. P. Mombaerts (Massachusetts Institute for Technology, Boston, MA; reference 37). All mice used were maintained under specific pathogen–free conditions in our animal facility. Animal care was in accordance with the guidelines of Chiba University.
Preparation of Liver Mononuclear Cells.
Liver mononuclear cells were isolated as previously described 38 39. In brief, the liver was pressed through stainless steel mesh (no. 200) and suspended in PBS. After washing once, the cells were resuspended in 33% Percoll solution containing heparin (100 U/ml), centrifuged at 2,000 rpm for 15 min at room temperature, and subjected to PCR and flow cytometry analyses.
Enrichment of NK1.1+ Cells by MACS.
Spleen cells were stained with FITC-conjugated anti-NK1.1 mAb and then cultivated with anti-FITC MicroBeads (no. 487-01; Miltenyi Biotec Inc.) for 15 min at 4°C. NK1.1+ cells were enriched by magnetic cell sorting according to the manufacturer's protocol (Miltenyi Biotec Inc.). About 45% of the MACS-purified cells were TCR-β+ NK1.1+ NKT cells. Most other populations were NK cells, which produce neither IL-4 nor IFN-γ upon Con A stimulation (see Fig. 4 A).
Figure 4.
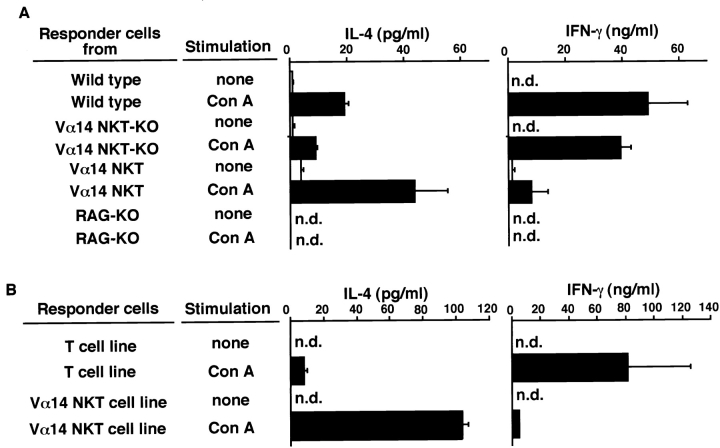
Production of IL-4 and IFN-γ from Con A–activated Vα14 NKT cells. Whole spleen cells from B6, Vα14 NKT KO, Vα14 NKT, and RAG KO mice (A) or the Vα14 NKT cell line and conventional T cell line (B) were stimulated in vitro with Con A (10 μg/ml). 24 h later, the amounts of IL-4 or IFN-γ in the culture supernatants were measured by ELISA. Mean values in triplicate samples with standard deviations are shown. n.d., not detectable.
Preparation of Vα14 NKT and T Cell Lines.
Spleen cells (4 × 104 per well) from Vα14 NKT mice or MACS-enriched NK1.1+ cells from B6, IL-4 KO, or IFN-γ KO mice were cultured in anti-CD3 mAb (2C11; 100 μg/ml)–coated 96-well plates (Falcon 3077) in the presence of 50 U/ml of recombinant mouse IL-2 for 3 d and further cultured for 2 d without anti-CD3 stimulation. More than 90% of the cultured cells from Vα14 NKT mice and ∼50% of the cultured cells from B6, IL-4 KO, or INF-γ KO mice were Vα14 NKT cells. T cell lines were also established under the same protocol as above with spleen T cells from Vα14 NKT KO mice.
Flow Cytometric Analysis.
Spleen cells (106) were incubated with anti-FcR (2.4G2; PharMingen), stained with mAbs (0.5 μg/ml) at 4°C for 30 min, and analyzed by EPICS XL-MCL (Beckman Coulter). FITC-conjugated anti–TCR-β (H57-597; PharMingen) and PE-conjugated anti-NK1.1 (PK136; PharMingen) mAbs were used to identify the NKT cell population. Anti–IL-4Rα (Genzyme Corp.), anti–common γ chain (PharMingen), and biotin-conjugated goat anti–rat Ig antibodies were used to stain the IL-4R complex. For IFN-γRα, biotinylated anti–IFN-γRα (PharMingen) was used. Dead cells were excluded by forward scatter, side scatter, and propidium iodide gating.
PCR.
To detect the rearranged Vα14Jα281 genes of migrated Vα14 NKT cells, genomic PCR was carried out on liver DNA from Vα14 NKT KO mice after transfer of Vα14 NKT cells by the method described in reference 40 using the following primers: 5′-CCGAATTCCCAAGTGGAGCAGAGTCCT-3′ and 5′-CCAAAATGCCTCCCTAA-3′. Reverse transcriptase–PCR was carried out on 1 μg of total RNA obtained from cultured Vα14 NKT cells as described 10 using the following primers: β-actin, 5′-GAGAGGGAAATCGTGCGTGA-3′ and 5′-ACATCTGCTGGAAGGTGGAC-3′; granzyme B, 5′-GCCCACAACATCAAAGAACAG-3′ and 5′-AACCAGCCACATAGCACACAT-3′; FasL, 5′-CTGGAATGGGAAGACACATA-3′ and 5′-AAAGGTCTTAGATTCCTCAA-3′; and TNF-α, 5′-ATGAGCACAGAAAGCATGATC-3′ and 5′-TACAGGCTTGTCACTCGAATT-3′.
Competitive PCR.
Competitor DNAs were constructed by PCR using a competitor DNA construction kit following the manufacturer's instructions (Takara Shuzo). A mixture of the cDNA and defined copy numbers of competitors was subjected to PCR amplification 40 using the primers described above. The ratio of the longer competitive product to the shorter target DNA product was used to estimate the amounts of target DNAs (490 vs. 454 bp for β-actin, 242 vs. 216 bp for granzyme B, 390 vs. 346 bp for FasL, and 342 vs. 276 bp for TNF-α).
Administration of Con A and Measurement of Serum Transaminase Activities.
Con A was purchased from Sigma Chemical Co. (cat. C0412). Either 0.5 mg (∼25 mg/kg) or 0.75 mg (∼37.5 mg/kg) of Con A was dissolved in 200 μl of pyrogen-free PBS and injected intravenously into mice. Serum activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured by Lippi-Guidi's method as described (Iatrozyme TA-LQ; Dia-Iatron; reference 41).
Adoptive Cell Transfer.
Vα14 NKT KO mice received spleen cells (2 × 107) from Vα14 NKT mice or splenic Vα14 NKT cells (5 × 106) isolated by MACS from B6, IL-4 KO, IFN-γ KO, gld/gld, or perforin KO mice. The Vα14 NKT cell line (2 × 106) was also used for cell transfer. 1 h after cell transfer, the mice were injected intravenously with Con A (0.5 mg).
Measurement of Cytokines in Culture Supernatants.
Spleen cells (106 per well) from B6, Vα14 NKT KO, or Vα14 NKT mice or the Vα14 NKT cell line and conventional T cell line were stimulated with Con A (10 μg/ml) for 24 h in 200-μl cultures. The amounts of IL-4 and IFN-γ in the culture supernatants were assayed using ELISA kits (EN-2601-80 and EN-2604-50; Endogen, Inc.) according to the manufacturer's protocol.
Histological Examination.
The livers from individual mice were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin for histological examination. Specimens were examined under a light microscope.
51Cr-Release Cytotoxic Assay.
The cytotoxic activity of Vα14 NKT cells upon activation with either IL-4 (100 U/ml; R & D Systems, Inc.) or IFN-γ (100 U/ml) for 8 h at 37°C was assayed by 4-h 51Cr-release cytotoxic assay on the TLR2 hepatocyte cell line (RIKEN Cell Bank) as described 42. The cells were harvested and seeded at the indicated E/T ratios (see Fig. 6 B).
Figure 6.
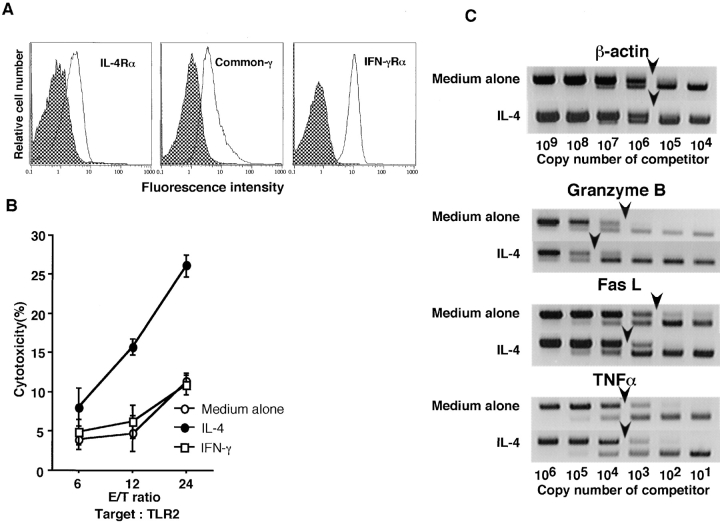
Augmentation of the cytotoxic activity of Vα14 NKT cells by short-term incubation with IL-4 in vitro. (A) Flow cytometric analysis of the expression of IL-4 and IFN-γRs on freshly isolated Vα14 NKT cells. (B) Spleen cells from Vα14 NKT mice were cultured for 8 h in the presence of 100 U/ml of IL-4 or 100 U/ml of IFN-γ. The cells were washed extensively and subjected to the standard 51Cr-release cytotoxic assay in triplicate on target TLR2 liver cells. (C) Augmented expression of mRNAs of granzyme B and FasL in Vα14 NKT cells upon stimulation with IL-4.
Results
Requirement of Vα14 NKT Cells for Con A–induced Hepatitis.
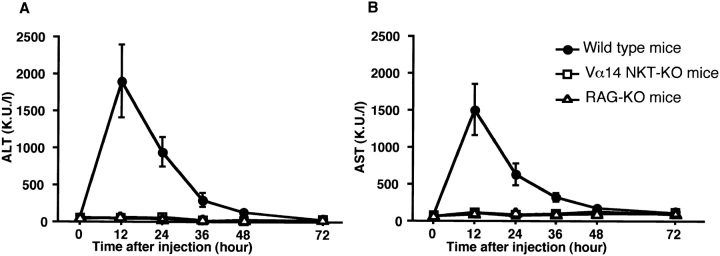
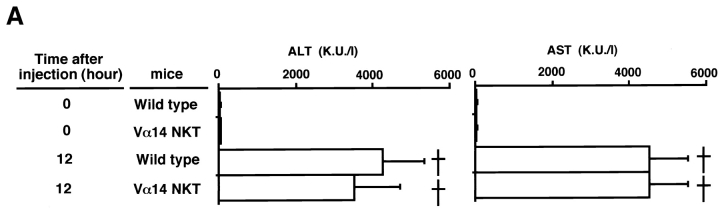
We investigated the role of Vα14 NKT cells in the development of Con A–induced hepatitis using Vα14 NKT KO mice lacking only Vα14 NKT cells. B6, Vα14 NKT KO, and RAG KO mice were injected intravenously with Con A, and the induction of hepatitis was evaluated by measuring the activities of two transaminases (ALT and AST) in the serum 12, 24, 36, 48, and 72 h after Con A treatment. As shown in Fig. 1 (A and B) the activities of both transaminases in B6 mice increased rapidly, reaching their peak values at 12 h. In contrast, no increase in the levels of either ALT or AST was detected in Vα14 NKT KO mice. These results strongly indicate that Vα14 NKT cells are required for hepatocyte injury mediated by Con A (Con A–induced hepatitis). In RAG KO mice, where only NK cells and no other lymphoid lineage cells develop, no increase in the level of either transaminase was detected, suggesting that NK cells are not essential.
Figure 1.
Elevation of serum transaminase activities after intravenous injection of Con A. Serum transaminase levels in B6 wild-type (•), Vα14 NKT KO (□), and RAG KO (▵) mice were assessed at different times after Con A (0.5 mg) administration. Three mice were used in each group. The mean transaminase activities (Karmen units per liter [K.U./l]) of ALT (A) and AST (B) of triplicate samples with standard errors are depicted.
Restoration of Con A–induced Hepatitis by Vα14 NKT Cell Transfer.
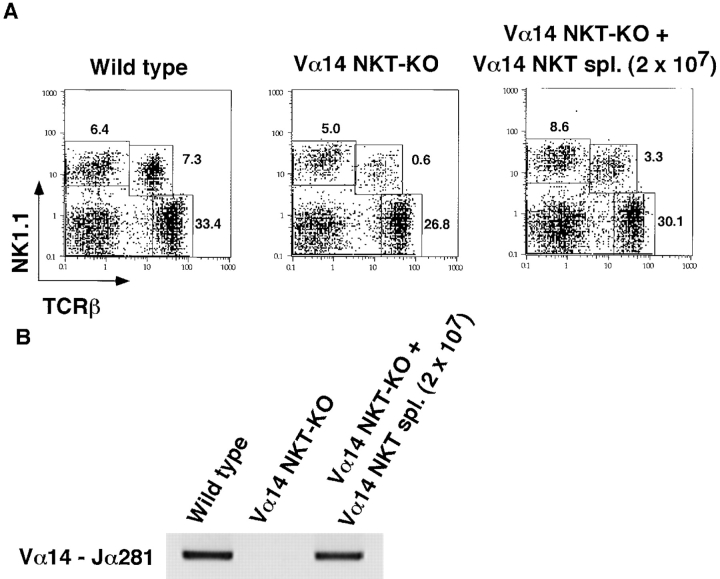
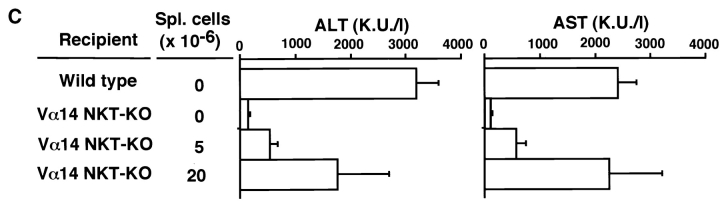
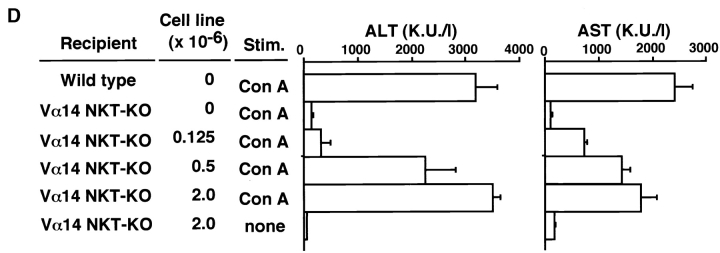
The requirement of Vα14 NKT cells for the development of hepatitis was further evaluated by experiments involving the adoptive transfer of Vα14 NKT cells into Vα14 NKT KO mice. 9 h after the intrasplenic injection of freshly isolated spleen cells (2 × 107) from Vα14 NKT mice, considerable numbers of transferred Vα14 NKT cells had migrated into the livers of recipient Vα14 NKT KO mice, as evidenced by the increased percentages of TCR-β+NK1.1+ NKT cells ( Fig. 2 A) and detectable amounts of rearranged Vα14Jα281 genes in the liver as assessed by genomic PCR ( Fig. 2 B).
Figure 2.
Restoration of Con A–induced hepatitis by Vα14 NKT cells in Vα14 NKT KO mice. (A) TCR-β/NK1.1 profiles of liver mononuclear cells of Vα14 NKT KO mice 9 h after adoptive transfer of 2 × 107 spleen cells from Vα14 NKT mice are shown. The percentages of cells present in each area are shown in each panel. (B) The rearranged Vα14Jα281 gene was assessed by genomic PCR with specific primers detecting Vα14Jα281. Vα14 NKT KO mice were intrasplenically injected with the indicated numbers of freshly prepared whole spleen cells from Vα14 NKT mice (C) or Vα14 NKT cell line established from spleen cells (spl.) of Vα14 NKT mice (D). 1 h after cell transfer, the mice were administered Con A (0.5 mg; Stim.) intravenously. The serum ALT and AST activities were measured 8 h after Con A injection. Three mice were used in each group, and the serum samples were analyzed individually. The mean values of triplicate samples with standard errors are shown.
As shown in Fig. 2 (C and D) the serum levels of the hepatic transaminases, ALT and AST, were significantly increased in Vα14 NKT KO mice receiving either freshly isolated Vα14 NKT cells ( Fig. 2 C) or Vα14 NKT cell line ( Fig. 2 D) in a dose-dependent manner. The intrasplenic injection of splenic Vα14 NKT cells (2 × 107) or Vα14 NKT cell line (2 × 106) resulted in almost full recovery. It was noted that neither intraperitoneal nor intravenous injection of Vα14 NKT cells induced any detectable elevation in the activities of these transaminases (data not shown). Thus, the intrasplenic route appears to be critical for the successful migration of Vα14 NKT cells into the recipient liver. Collectively, Vα14 NKT cells, together with the administration of Con A, are essential for the induction of Con A–induced hepatitis.
An Indispensable Role of Vα14 NKT Cells in the Development of Con A–induced Hepatitis.
It is of interest whether Vα14 NKT cells are the final effector cells or the inducer cells in Con A–induced hepatitis. To address this question, we used Vα14 NKT mice that have only Vα14 NKT cells and no T, B, or NK cells. A dramatic elevation of serum transaminases was observed in Vα14 NKT mice, and all mice died within 24 h when 0.75 mg of Con A was used ( Fig. 3). The transaminase elevation was not reproducibly observed in Vα14 NKT mice receiving a regular dose of Con A (0.5 mg; data not shown). This observation suggests that other cell components, such as T cells, are also involved, and that the hepatitis is induced more efficiently in wild-type than in Vα14 NKT mice. Thus, we used 0.75 mg of Con A in the experiments with Vα14 NKT mice.
Figure 3.
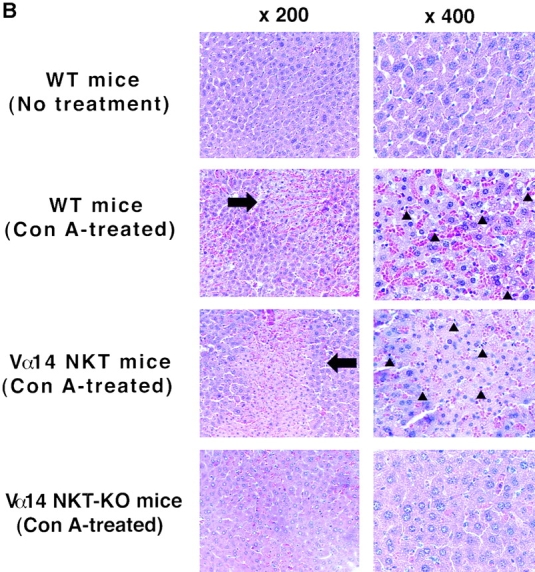
Development of Con A–induced hepatitis in Vα14 NKT mice. (A) Serum transaminase levels in B6 and Vα14 NKT mice 12 h after Con A administration (0.75 mg). All mice died within 24 h (†). The mean values of three samples with standard errors are shown. (B) Light micrographs of the liver with hematoxylin and eosin staining (×200 and ×400) are shown. The indicated mice were treated with 0.75 mg of Con A and killed 8 h later. Arrows indicate massive necrosis observed in the liver; arrowheads indicate mononuclear cell infiltration. WT, wild-type.
We carried out histological examinations of the liver 8 h after the administration of 0.75 mg of Con A ( Fig. 3 B). In B6 mice, a severe bridging necrosis was observed in the area between the central veins and the portal tracts (×200; arrow). In addition, photographs at higher magnification (400) revealed the infiltration of mononuclear cells (arrowheads) and Kupffer cells within sinusoids adjacent to the degenerated hepatocytes. Massive red blood cells were also observed in the sinusoidal area. Similar histological findings were observed in the livers of Vα14 NKT mice. In contrast, no significant histological changes were observed in Vα14 NKT KO mice. Essentially similar histological findings were observed in B6 mice receiving 0.5 mg of Con A (data not shown). These results indicate that Con A–induced hepatitis is evoked by Vα14 NKT cells even in the absence of conventional T, B, and NK cells.
Preferential Production of IL-4 by Con A–activated Vα14 NKT Cells.
To address the molecular mechanisms underlying Vα14 NKT cell–mediated hepatocyte injury, we first assessed the production of cytokines from Vα14 NKT cells stimulated with Con A. This is because Vα14 NKT cells are known to produce both IL-4 and IFN-γ very rapidly when they are stimulated with anti-CD3 mAb 13 14 or the ligand α-GalCer 9. Spleen cells from B6, Vα14 NKT KO, Vα14 NKT, and RAG KO mice were stimulated in vitro with 10 μg/ml of Con A, and the amounts of IL-4 and IFN-γ produced in the culture supernatants were assessed by ELISA. As shown in Fig. 4 A, spleen cells from Vα14 NKT mice produced levels of IL-4 several times higher than those produced by B6 or Vα14 NKT KO mice. In addition, unexpectedly low amounts of IFN-γ were produced after stimulation with Con A. In RAG KO mice bearing only NK cells, as expected, no IL-4 or IFN-γ was produced, suggesting that NK cells are not the producers of these cytokines upon Con A stimulation ( Fig. 4 A). These results suggest that Vα14 NKT cells produce large amounts of IL-4 upon Con A stimulation. Similar results were obtained using the Vα14 NKT cell line ( Fig. 4 B).
Requirement of IL-4 but not IFN-γ Produced by Vα14 NKT Cells for the Development of Con A–induced Hepatitis.
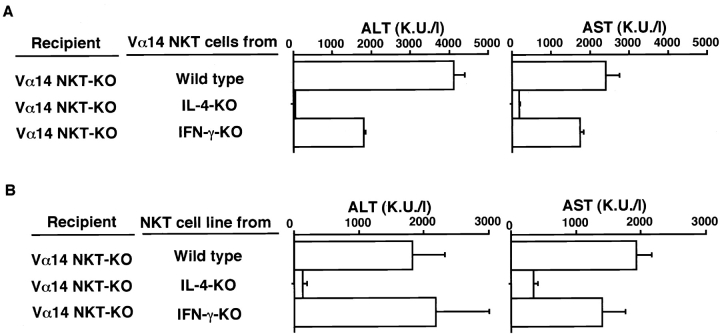
In the next experiments, the involvement of IL-4 and IFN-γ produced by Vα14 NKT cells in Con A–induced hepatitis was examined in vivo. Vα14 NKT cells were enriched from spleens of B6, IL-4 KO, and IFN-γ KO mice by MACS using anti-NK1.1 mAb. The cells were adoptively transferred into Vα14 NKT KO mice ( Fig. 5 A). Similarly, the Vα14 NKT cell line was transferred into Vα14 NKT KO mice ( Fig. 5 B). The mice were then injected intravenously with Con A, and ALT and AST transaminase activities were measured 8 h later. As shown in Fig. 5Vα14 NKT cells derived from IL-4 KO mice did not restore hepatitis, whereas those from IFN-γ KO mice elicited a significant elevation in serum transaminase activities to levels equivalent to those induced by Vα14 NKT cells from B6 mice. Vα14 NKT cells from IL-4 KO mice produced levels of IFN-γ equivalent to those of B6 mice (data not shown). These results clearly indicate that IL-4 but not IFN-γ produced by Vα14 NKT cells is required for the development of Con A–induced hepatitis.
Figure 5.
Requirement of IL-4 produced by Vα14 NKT cells for Con A–induced hepatitis. (A) MACS-sorted NK1.1+ spleen cells (107) from B6, IL-4 KO, or IFN-γ KO mice were transferred intrasplenically into Vα14 NKT KO mice. 1 h later, the mice were injected with Con A (0.5 mg). The serum activities of ALT and AST were assessed 8 h after Con A injection. (B) Induction of Con A–induced hepatitis by adoptive transfer of cells from the Vα14 NKT cell line (2 × 106). Three mice were used in each group. The mean values in triplicate with standard errors are shown.
Enhancement of Vα14 NKT Cell–mediated Cytotoxicity upon Stimulation with IL-4.
The next experiments were designed to clarify the effector mechanism of IL-4 in the development of Con-A–induced hepatitis. First, the effect of IL-4 on Vα14 NKT cell–mediated cytotoxicity was assessed. Significant levels of IL-4R (both α chain and common γ chain) as well as IFN-γR were expressed on the freshly isolated Vα14 NKT cells ( Fig. 6 A). Consequently, freshly prepared Vα14 NKT cells were incubated with 100 U/ml of recombinant IL-4 for 8 h in vitro. The effect of IFN-γ was also examined. To our surprise, the cytotoxic activity of Vα14 NKT cells against the TLR2 hepatocyte cell line was significantly enhanced when Vα14 NKT cells were treated with IL-4 but not with IFN-γ ( Fig. 6 B). Similar results were obtained in experiments using other target cells, such as YAC-1 (data not shown). Consequently, we examined the expression of granzyme B, FasL, and TNF-α in IL-4–stimulated Vα14 NKT cells by competitive PCR assay. The FasL and granzyme B transcripts but not TNF-α transcripts in Vα14 NKT cells were significantly enhanced (∼10-fold) by treatment with IL-4 ( Fig. 6 C).
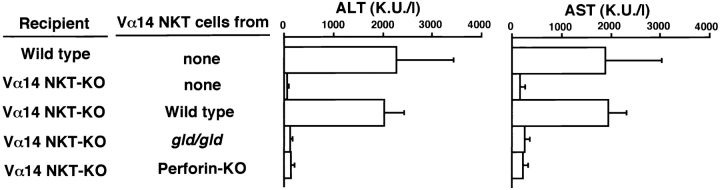
The role of these molecules produced or expressed by Vα14 NKT cells in vivo was assessed by cell transfer experiments. As shown in Fig. 7Vα14 NKT cells isolated from FasL-mutant gld/gld mice or perforin KO mice failed to restore the induction of hepatitis ( Fig. 7). Thus, both perforin and FasL are essential for the development of this hepatitis. Taken with the results shown in Fig. 6, it is most likely that the Con A–stimulated Vα14 NKT cells release IL-4, which in turn acts on Vα14 NKT cells to enhance their cytotoxic activity by upregulating the expressions of granzyme B and FasL.
Figure 7.
Requirement of FasL expression and perforin production from Vα14 NKT cells for Con A–induced hepatitis. MACS-sorted NK1.1+ spleen cells (107) from B6, B6-gld/gld, or perforin KO mice were transferred intrasplenically into Vα14 NKT KO mice. 1 h later, the mice were injected with Con A (0.5 mg). 8 h later, the serum activities of ALT and AST were assessed. The mean values of triplicate samples with standard errors are shown.
Discussion
It has been suggested that CD4+ T cells 23 24 and NK1.1+ T cells 25 are involved in the development of Con A–induced hepatitis, although the precise cellular and molecular requirements have not been clarified. We demonstrate here that Vα14 NKT cells, a novel lymphocyte lineage, are required and sufficient for the development of hepatitis. Vα14 NKT mice possessing only Vα14 NKT cells exhibited a dramatic increase in serum transaminase levels after injection of Con A, and all mice died within 24 h ( Fig. 3 A). In sharp contrast, Vα14 NKT KO mice lacking only Vα14 NKT cells did not develop hepatocyte injury ( Fig. 1) or mononuclear cell infiltration into the liver ( Fig. 3 B). Thus, Vα14 NKT cells are able to function as effector cells to develop Con A–induced hepatitis in the absence of conventional T cells.
In this study, the involvement of CD4+ T cells in the disease development reported by several investigators 23 24 25 26 27 28 29 30 31 32 33 34 is not formally addressed. However, T cells seem to be clearly involved in the development of this hepatitis, because the hepatitis occurs more efficiently in the presence of both CD4+ T cells and Vα14 NKT cells than Vα14 NKT cells alone. In our experimental system, Vα14 NKT mice lacking T cells require 0.75 mg of Con A for the reproducible induction of hepatitis, whereas 0.5 mg of Con A is sufficient in wild-type mice ( Fig. 1 and Fig. 3 A).
It is interesting to note that the level of transaminase elevation depends on the number of Vα14 NKT cells transferred ( Fig. 2 C). The hepatitis induction was restored by transfer of freshly isolated Vα14 NKT cells or Vα14 NKT cell lines in a dose-dependent fashion. These findings are also confirmed in physiological situations. For example, SJL/J mice are known to have significantly lower numbers of NKT (TCR-β+IL-2Rβ+) cells compared with other congenic mice 43 44 and indeed were relatively resistant to Con A–induced hepatitis (data not shown). In addition, young mice, such as 2-wk-old B6 mice, had a few NKT cells (∼30% of adult levels; reference 45) and were considerably resistant to Con A (data not shown). Collectively, in normal wild-type mice, the administration of Con A appears to induce a destructive immune response leading to hepatocyte injury, which is probably initiated and mediated by Vα14 NKT cells and exacerbated by conventional T cells.
More interestingly, IL-4 produced by Con A–stimulated Vα14 NKT cells appears to play an indispensable role in disease development by enhancing the cytotoxic activity of Vα14 NKT cells. In fact, freshly isolated Vα14 NKT cells express significant levels of IL-4R (IL-4Rα and common γ chains) on their cell surfaces ( Fig. 6 A), and the short-term treatment of Vα14 NKT cells with IL-4 results in a dramatic enhancement of cytotoxic activity against various target cells, including a hepatocyte cell line ( Fig. 6 B). The enhanced cytotoxic activity induced by IL-4 accompanied increases in the levels of granzyme B and FasL mRNAs ( Fig. 6 C). These results are in good agreement with findings that Con A–induced hepatitis is significantly inhibited in IL-4 KO mice (data not shown) or by pretreatment with anti–IL-4 mAb 25 and that Vα14 NKT cells from IL-4 KO mice do not initiate Con A–induced hepatitis ( Fig. 5). Thus, it is most likely that the IL-4 produced by Con A–activated Vα14 NKT cells acts on Vα14 NKT cells in an autocrine fashion and induces upregulation of granzyme B and FasL transcripts, resulting in an enhancement of Vα14 NKT cell–mediated cytotoxicity.
Several cytotoxic effector molecules are considered to be involved in the development of Con A–induced hepatitis. First is the perforin–granzyme system that is known to induce hepatocyte injury 29. Indeed, perforin KO mice 29 and Vα14 NKT cells from perforin KO mice fail to develop Con A–induced hepatitis ( Fig. 7), suggesting that the perforin–granzyme system is essential for the induction of Vα14 NKT cell–mediated hepatitis.
Although the involvement of the Fas–FasL system in the induction of Con A–induced hepatitis is controversial 26 27 28 29 34, the system is likely to be one of the effector mechanisms for hepatocyte injury, particularly for Vα14 NKT cell–mediated hepatocyte injury. In fact, this hepatitis has been reported to be significantly milder in Fas-mutant lpr/lpr mice 26 28, and it is completely abrogated in gld/gld mice in which FasL is defective 27 28 or in mice pretreated with anti-FasL antibody 28. It is also well known that the stimulation of Fas on hepatocytes by anti-Fas antibody causes severe damage to hepatocytes by apoptotic cell death 46. In addition, normal hepatocytes express a significant amount of Fas 46, and the expression level of Fas mRNA in hepatocytes is increased upon Con A stimulation 26. As shown in Fig. 6 C, levels of FasL mRNA are significantly upregulated by IL-4 treatment. Thus, it is conceivable that a direct interaction between IL-4–stimulated Vα14 NKT cells expressing high levels of FasL and Con A–stimulated hepatocytes with an augmented expression of Fas results in the induction of apoptotic cell death in hepatocytes. Moreover, as demonstrated in Fig. 7, cell transfer of Vα14 NKT cells obtained from FasL-mutant gld/gld mice fails to restore the induction of Con A–induced hepatitis. This result indicates that the Fas–FasL system is crucial for Vα14 NKT cell–mediated hepatocyte injury.
In addition to the contribution of perforin and FasL as effector molecules in Vα14 NKT cell–mediated hepatitis, TNF-α and IFN-γ produced by non-NKT cells such as macrophages and CD4+ T cells, respectively, are reported to play crucial roles in the development of Con A–induced hepatitis. It is demonstrated that mice pretreated with anti–mouse TNF-α antiserum 31 or TNF-α inhibitor (TNF binding protein; reference 34) or mice deficient for TNFR1 and TNFR2 are resistant to Con A–induced hepatitis 33 and that the enhanced TNF-α production after Con A stimulation appears to be produced mainly by macrophages in the liver 32. In fact, TNF-α mRNA expression in Vα14 NKT cells is unchanged even after IL-4 treatment ( Fig. 6 C).
IFN-γ also seems to be important for the development of Con A–induced hepatitis, because mice pretreated with anti–IFN-γ antiserum 30 or IFN-γ–deficient mice 26 are resistant to Con A–induced hepatitis. For IFN-γ production, CD4+ T cells seem to be responsible, because Con A–activated T cells produce large amounts of IFN-γ, and Vα14 NKT cells produce a little ( Fig. 4). It is likely that IFN-γ directly acts on hepatocytes and induces hepatocyte injury, as hepatocytes express IFN-γR on the cell surface 47. It is also interesting to note that IFN-γ upregulates TNFRs on the hepatocyte cell surface 48 49. This could be the reason for the enhancing effects of IFN-γ on TNF-induced apoptosis 30. Thus, synergistic effects of IFN-γ and TNF-α produced by CD4+ T cells and macrophages may accelerate apoptosis in hepatocytes.
Con A–induced hepatitis is thought to be a model of immunologically induced hepatocyte injury, and its histological features resemble those of viral or drug-induced acute hepatitis in humans 50 51. The function of Vα24Vβ11 NKT cells in humans, a counterpart of mouse Vα14 NKT cells, is highly conserved, e.g., sharing ligand specificity and CD1d restriction 52 53 54; these cells also possess potent cytotoxic activity against a wide variety of tumor cells 55. Thus, it is easy to speculate that Vα24Vβ11 NKT cells play a critical role in certain types of acute hepatitis in humans. More recently, it has been demonstrated that Vα14 NKT cells are activated by parasite glycosylphosphatidylinositol 12 or bacterial LPS 56, indicating their physiological roles in the immune responses to microorganisms. This suggests that murine Vα14 NKT cells or human Vα24 NKT cells are involved in the development of various diseases or regulation of immune responses after microbial infections.
Acknowledgments
We thank Ms. Hiroko Tanabe for preparation of this manuscript.
1 Abbreviations used in this paper: KO, knock-out; L, ligand; RAG, recombination activating gene.
References
- Lantz O., Bendelac A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I–specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 1994;180:1097–1106 . doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino Y., Kanno R., Ito T., Higashino K., Taniguchi M. Predominant expression of invariant Vα14+ TCR α chain in NK1.1+ T cell populations. Int. Immunol. 1995;7:1157–1161 . doi: 10.1093/intimm/7.7.1157. [DOI] [PubMed] [Google Scholar]
- Budd R.C., Miescher G.C., Howe R.C., Lees R.K., Bron C., MacDonald H.R. Developmentally regulated expression of T cell receptor β chain variable domains in immature thymocytes. J. Exp. Med. 1987;166:577–582 . doi: 10.1084/jem.166.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes B.J., Kruisbeek A.M., Ton-That H., Weston M.A., Coligan J.E., Schwartz R.H., Pardoll D.M. A novel population of T-cell receptor αβ-bearing thymocytes which predominantly expresses a single Vβ gene family. Nature. 1987;329:251–254 . doi: 10.1038/329251a0. [DOI] [PubMed] [Google Scholar]
- Sykes M. Unusual T cell populations in adult murine bone marrow. Prevalence of CD3+CD4−CD8− and αβTCR+NK1.1+ cells. J. Immunol. 1990;145:3209–3215 . [PubMed] [Google Scholar]
- Levitsky H.I., Golumbek P.T., Pardoll D.M. The fate of CD4−8− T cell receptor-αβ+ thymocytes. J. Immunol. 1991;146:1113–1117 . [PubMed] [Google Scholar]
- Arase H., Arase N., Ogasawara K., Good R.A., Onoe K. An NK1.1+ CD4+8− single-positive thymocyte subpopulation that expresses a highly skewed T-cell antigen receptor Vβ family. Proc. Natl. Acad. Sci. USA. 1992;89:6506–6510 . doi: 10.1073/pnas.89.14.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Shin T., Kawano T., Sato H., Kondo E., Toura I., Kaneko Y., Koseki H., Kanno M., Taniguchi M. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626 . doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Motoki K., Ueno H., Nakagawa R., Sato H., Kondo E. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629 . doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- Makino Y., Kanno R., Koseki H., Taniguchi M. Development of Vα14+ NK T cells in the early stages of embryogenesis. Proc. Natl. Acad. Sci. USA. 1996;93:6516–6520 . doi: 10.1073/pnas.93.13.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Sato H., Kondo E., Harada M., Koseki H., Nakayama T. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells. Proc. Natl. Acad. Sci. USA. 1998;95:5690–5693 . doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L., McConville M.J., Hansen D., Campbell A.S., Fraser-Reid B., Grusby M.J., Tachado S.D. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283:225–229 . doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- Arase H., Arase N., Nakagawa K., Good R.A., Onoe K. NK1.1+ CD4+ CD8− thymocytes with specific lymphokine secretion. Eur. J. Immunol. 1993;23:307–310 . doi: 10.1002/eji.1830230151. [DOI] [PubMed] [Google Scholar]
- Chen H., Paul W.E. Cultured NK1.1+ CD4+ T cells produce large amounts of IL-4 and IFN-γ upon activation by anti-CD3 or CD1. J. Immunol. 1997;159:2240–2249 . [PubMed] [Google Scholar]
- Cui J., Wanatabe N., Kawano T., Yamashita M., Kamata T., Shimizu C., Kimura M., Shimizu E., Koike J., Koseki H. Inhibition of T helper cell type 2 differentiation and immunoglobulin E response by ligand-activated Vα14 natural killer T cells. J. Exp. Med. 1999;190:783–792 . doi: 10.1084/jem.190.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieza M.A., Ito T., Cui J., Makino Y., Kawano T., Tsuchida K., Koike T., Shirai T., Yagita H., Matsuzawa A. Selective reduction of Vα14+ NK T cells associated with disease development in autoimmune-prone mice. J. Immunol. 1996;156:4035–4040 . [PubMed] [Google Scholar]
- Gombert J.M., Herbelin A., Tancrede-Bohin E., Dy M., Carnaud C., Bach J.F. Early quantitative and functional deficiency of NK1+-like thymocytes in the NOD mouse. Eur. J. Immunol. 1996;26:2989–2998 . doi: 10.1002/eji.1830261226. [DOI] [PubMed] [Google Scholar]
- Baxter A.G., Kinder S.J., Hammond K.J.L., Scollay R., Godfrey D.I. Association between αβTCR+CD4− CD8− T-cell deficiency and IDDM in NOD/Lt mice. Diabetes. 1997;46:572–582 . doi: 10.2337/diab.46.4.572. [DOI] [PubMed] [Google Scholar]
- Lehuen A., Lantz O., Beaudoin L., Laloux V., Carnaud C., Bendelac A., Bach J.F., Monteiro R.C. Overexpression of natural killer T cells protects Vα14-Jα281 transgenic nonobese diabetic mice against diabetes. J. Exp. Med. 1998;188:1831–1839 . doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigaki M., Nishimura H., Naiki Y., Yoshioka K., Kawano T., Tanaka Y., Taniguchi M., Kakumu S., Yoshikai Y. The roles of intrahepatic Vα14+ NK1.1+ T cells for liver injury induced by Salmonella infection in mice. Hepatology. 1999;29:1799–1808 . doi: 10.1002/hep.510290605. [DOI] [PubMed] [Google Scholar]
- Wilson S.B., Kent S.C., Patton K.T., Orban T., Jackson R.A., Exley M., Porcelli S., Schatz D.A., Atkinson M.A., Balk S.P. Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature. 1998;391:177–181 . doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- Sumida T., Sakamoto A., Murata H., Makino Y., Takahashi H., Yoshida S., Nishioka K., Iwamoto I., Taniguchi M. Selective reduction of T cells bearing invariant Vα24JαQ antigen receptor in patients with systemic sclerosis. J. Exp. Med. 1995;182:1163–1168 . doi: 10.1084/jem.182.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiegs G., Hentschel J., Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J. Clin. Invest. 1992;90:196–203 . doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuhara H., O'Neil E., Seki N., Ogawa T., Kusunoki C., Otsuka K., Satoh S., Niwa M., Senoh H., Fujiwara H. T cell activation–associated hepatic injurymediation by tumor necrosis factors and protection by interleukin 6. J. Exp. Med. 1994;179:1529–1537 . doi: 10.1084/jem.179.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyabe S., Seki S., Iiai T., Takeda K., Shirai K., Watanabe H., Hiraide H., Uchiyama M., Abo T. Requirement of IL-4 and liver NK1+ T cells for concanavalin A-induced hepatic injury in mice. J. Immunol. 1997;159:1537–1542 . [PubMed] [Google Scholar]
- Tagawa Y., Sekikawa K., Iwakura Y. Suppression of concanavalin A-induced hepatitis in IFN-γ−/− mice, but not in TNF-α−/− mice. Role for IFN-γ in activating apoptosis of hepatocytes. J. Immunol. 1997;159:1418–1428 . [PubMed] [Google Scholar]
- Tagawa Y., Kakuta S., Iwakura Y. Involvement of Fas/Fas ligand system-mediated apoptosis in the development of concanavalin A-induced hepatitis. Eur. J. Immunol. 1998;28:4105–4113 . doi: 10.1002/(SICI)1521-4141(199812)28:12<4105::AID-IMMU4105>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Seino K., Kayagaki N., Takeda K., Fukao K., Okumura K., Yagita H. Contribution of Fas ligand to T cell-mediated hepatic injury in mice. Gastroenterology. 1997;113:1315–1322 . doi: 10.1053/gast.1997.v113.pm9322527. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Morita M., Akaike T. Concanavalin A induces perforin-mediated but not Fas-mediated hepatic injury. Hepatology. 1996;24:702–710 . doi: 10.1053/jhep.1996.v24.pm0008781346. [DOI] [PubMed] [Google Scholar]
- Kusters S., Gantner F., Kunstle G., Tiegs G. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462–471 . doi: 10.1053/gast.1996.v111.pm8690213. [DOI] [PubMed] [Google Scholar]
- Gantner F., Leist M., Lohse A.W., Germann P.G., Tiegs G. Concanavalin A-induced T-cell-mediated hepatic injury in micethe role of tumor necrosis factor. Hepatology. 1995;21:190–198 . doi: 10.1016/0270-9139(95)90428-x. [DOI] [PubMed] [Google Scholar]
- Gantner F., Leist M., Kusters S., Vogt K., Volk H.D., Tiegs G. T cell stimulus-induced crosstalk between lymphocytes and liver macrophages results in augmented cytokine release. Exp. Cell Res. 1996;229:137–146 . doi: 10.1006/excr.1996.0351. [DOI] [PubMed] [Google Scholar]
- Kusters S., Tiegs G., Alexopoulou L., Pasparakis M., Douni E., Kunstle G., Bluethmann H., Wendel A., Pfizenmaier K., Kollias G. In vivo evidence for a functional role of both tumor necrosis factor (TNF) receptors and transmembrane TNF in experimental hepatitis. Eur. J. Immunol. 1997;27:2870–2875 . doi: 10.1002/eji.1830271119. [DOI] [PubMed] [Google Scholar]
- Ksontini R., Colagiovanni D.B., Josephs M.D., Edwards C.K., III, Tannahill C.L., Solorzano C.C., Norman J., Denham W., Clare-Salzler M., MacKay S.L.D. Disparate roles for TNF-α and Fas ligand in concanavalin A-induced hepatitis. J. Immunol. 1998;160:4082–4089 . [PubMed] [Google Scholar]
- Kopf M., Gros G.L., Bachmann M., Lamers M.C., Bluethmann H., Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248 . doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- Kagi D., Ledermann B., Burki K., Seiler P., Odermatt B., Olsen K.J., Podack E.R., Zinkernagel R.M., Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37 . doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R.S., Herrup K., Tonegawa S., Papaioannou V.E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877 . doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Goossens P.L., Jouin H., Marchal G., Milon G. Isolation and flow cytometric analysis of the free lymphomyeloid cells present in murine liver. J. Immunol. Methods. 1990;132:137–144 . doi: 10.1016/0022-1759(90)90407-m. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Ohtsuka K., Kimura M., Ikarashi Y., Ohmori K., Kusumi A., Ohteki T., Seki S., Abo T. Details of an isolation method for hepatic lymphocytes in mice. J. Immunol. Methods. 1992;146:145–154 . doi: 10.1016/0022-1759(92)90223-g. [DOI] [PubMed] [Google Scholar]
- Sato H., Nakayama T., Tanaka Y., Yamashita M., Shibata Y., Kondo E., Saito Y., Taniguchi M. Induction of differentiation of pre-NKT cells to mature Vα14 NKT cells by granulocyte/macrophage colony-stimulating factor. Proc. Natl. Acad. Sci. USA. 1999;96:7439–7444 . doi: 10.1073/pnas.96.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi U., Guidi G. A new colorimetric ultramicromethod for serum glutamic-oxalacetic and glutamic-pyruvic transaminase determination. Clin. Chim. Acta. 1970;28:431–437 . doi: 10.1016/0009-8981(70)90069-0. [DOI] [PubMed] [Google Scholar]
- Yanai N., Suzuki M., Obinata M. Hepatocyte cell lines established from transgenic mice harboring temperature-sensitive simian virus 40 large T-antigen gene. Exp. Cell Res. 1991;197:50–56 . doi: 10.1016/0014-4827(91)90478-d. [DOI] [PubMed] [Google Scholar]
- Beutner U., Launois P., Ohteki T., Louis J.A., MacDonald H.R. Natural killer-like T cells develop in SJL mice despite genetically distinct defects in NK1.1 expression and in inducible interleukin-4 production. Eur. J. Immunol. 1997;27:928–934 . doi: 10.1002/eji.1830270419. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Bendelac A., Hu-Li J., Paul W.E. Defective IgE production by SJL mice is linked to the absence of CD4+, NK1.1+ T cells that promptly produce interleukin 4. Proc. Natl. Acad. Sci. USA. 1995;92:11931–11934 . doi: 10.1073/pnas.92.25.11931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseki H., Imai K., Nakayama F., Sado T., Moriwaki K., Taniguchi M. Homogenous junctional sequence of the V14+ T-cell antigen receptor α chain expanded in unprimed mice. Proc. Natl. Acad. Sci. USA. 1990;87:5248–5252 . doi: 10.1073/pnas.87.14.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara J., Watanabe-Fukunaga R., Adachi M., Matsuzawa A., Kasugai T., Kitamura Y., Itoh N., Suda T., Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809 . doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Boehm U., Klamp T., Groot M., Howard J.C. Cellular responses to interferon-γ. Annu. Rev. Immunol. 1997;15:749–795 . doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- Aggarwal B.B., Eessalu T.E., Hass P.E. Characterization of receptors for human tumour necrosis factor and their regulation by γ-interferon. Nature. 1985;318:665–667 . doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- Ruggiero V., Tavernier J., Fiers W., Baglioni C. Induction of the synthesis of tumor necrosis factor receptors by interferon-γ. J. Immunol. 1986;136:2445–2450 . [PubMed] [Google Scholar]
- Boyer J.L. The diagnosis and pathogenesis of clinical variants in viral hepatitis. Am. J. Clin. Pathol. 1976;65:898–908 . [PubMed] [Google Scholar]
- Horney J.T., Galambos J.T. The liver during and after fulminant hepatitis. Gastroenterology. 1977;73:639–645 . [PubMed] [Google Scholar]
- Brossay L., Chioda M., Burdin N., Koezuka Y., Casorati G., Dellabona P., Kronenberg M. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 1998;188:1521–1528 . doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spada F.M., Koezuka Y., Porcelli S.A. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J. Exp. Med. 1998;188:1529–1534 . doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T., Tanaka Y., Shimizu E., Kaneko Y., Kamata N., Sato H., Osada H., Sekiya S., Nakayama T., Taniguchi M. A novel recognition motif of human NKT antigen receptor for a glycolipid ligand. Int. Immunol. 1999;11:881–887 . doi: 10.1093/intimm/11.6.881. [DOI] [PubMed] [Google Scholar]
- Kawano T., Nakayama T., Kamada N., Kaneko Y., Harada M., Ogura N., Akutsu Y., Motohashi S., Iizasa T., Endo H. Ant-tumor cytotoxicity mediated by ligand-activated human Vα24 NKT cells. Cancer Res. 1999;59:5102–5105 . [PubMed] [Google Scholar]
- Takahashi M., Ogasawara K., Takeda K., Hashimoto W., Sakihara H., Kumagai K., Anzai R., Satoh M., Seki S. LPS induces NK1.1+ αβ T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kupffer cells. J. Immunol. 1996;156:2436–2442. [PubMed] [Google Scholar]