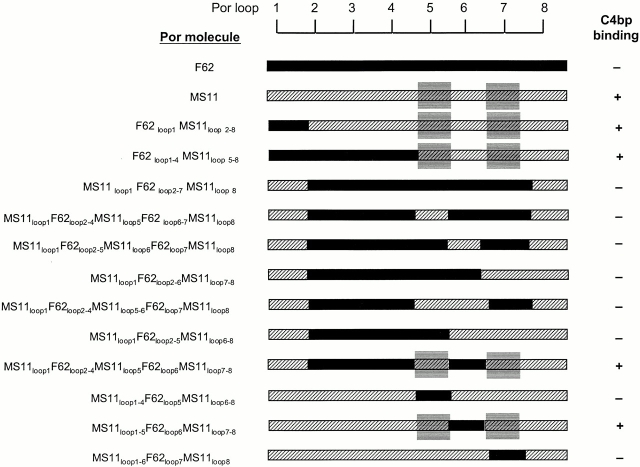
Figure 4.
MS11 Por1B loops 5 and 7 together participate in forming a C4bp-binding region. Transformation of F62 with pUNCH61 (reference 40) resulted in strains with hybrid Por molecules F62loop1MS11loop2–8 and F62loop1–4MS11loop5–8. F62 sequence is indicated by black bars; MS11 sequence is represented by hatched bars. Both hybrids bound C4bp, suggesting that the C4bp binding region in MS11 lay in a region encompassed by loops 5–8. The BbsI-BsgI fragment of F62 was cloned into pUNCH61 to obtain pBUMC1 and this plasmid was used to transform F62. This yielded a gonococcal strain with hybrid Por molecule MS11loop1 F62loop2–7MS11loop1–8, which did not bind C4bp, thus eliminating MS11 loop 8 as the C4bp binding loop. Using overlap extension PCR or site-directed mutagenesis on pBUMC1, we mutated loops 5, 6, or 7 either individually or in combination and then transformed F62. We noted that only hybrid Por molecules that contained both MS11 loop 5 and loop 7 bound C4bp. To further demonstrate the absolute requirement of MS11 loops 5 and 7, we mutated MS11 loops 5, 6, and 7 individually in pUNCH61 to resemble the corresponding F62 loop, and then transformed F62. Mutations of loop 5 (MS11loop1–4F62loop5MS11loop6–8) and loop 7 (MS11loop1–6F62loop7MS11loop8) resulted in complete abrogation of C4bp binding, whereas mutating loop 6 (MS11loop1–5F62loop6MS11loop7–8) did not influence C4bp binding. Collectively, these data suggest that MS11 loops 5 and 7 together participate in forming a C4bp-binding domain.