Figure 7.
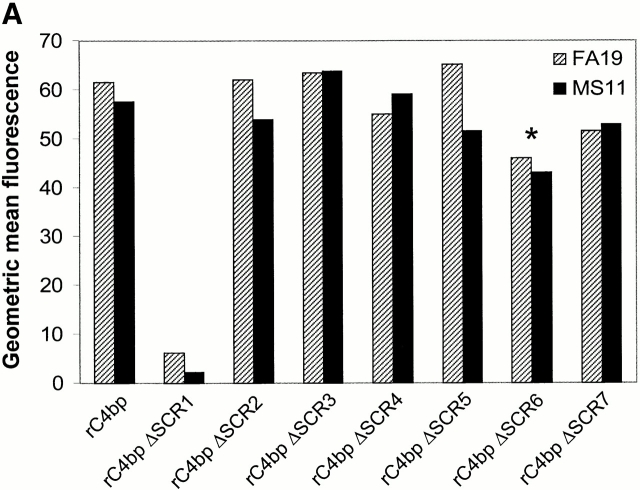
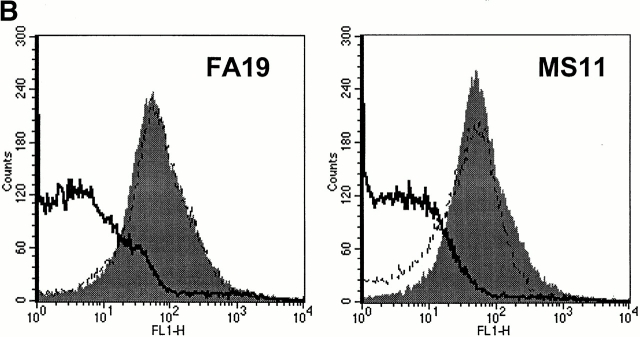
C4bp SCR 1 contains both Por1A as well as Por1B binding regions. (A) Mapping of Por binding sites in C4bp. Binding of recombinant C4bp (rC4bp) and seven rC4bp mutant molecules lacking individual SCRs to FA19 (Por1A) and MS11 (Por1B) were studied using flow cytometry. 108 organisms were incubated with 2.5 μg of each rC4bp molecule, followed by detection with sheep polyclonal anti–human C4bp and disclosed with anti–sheep IgG-FITC. All rC4bp molecules that contained SCR 1 bound both FA19 as well as MS11; only the mutant that lacked SCR 1 (rC4bp ΔSCR1) did not bind either strain. Because artifactually lower fluorescence (threefold less binding compared with rC4bp) was observed when rC4bp ΔSCR6 was detected with the polyclonal anti-C4bp Ab, we used anti-C4bp mAb (Quidel Corp.) and mAb 67 (specific for SCR 4) to detect rC4bp ΔSCR6 bound to both strains. Compared with rC4bp, rC4bp ΔSCR6 bound with similar fluorescence intensities, when either mAb was used as a probe. Binding of rC4bp ΔSCR6 to both strains using the Quidel Corp. mAb is shown here (indicated by the asterisk). rC4bp ΔSCR1 did not bind to either strains when mAb 67 or the Quidel Corp. anti-C4bp mAb was used as the detection Ab. (B) Anti-C4bp SCR1 mAb blocks C4bp binding to gonococci. Further evidence that SCR 1 contains Por1A and Por1B binding regions was provided because mAb 104 that is specific for C4bp SCR 1 completely inhibits binding of C4bp to both FA19 (Por1A) as well as MS11 (Por1B; solid lines); control mAb 67 (specific for the irrelevant C4bp SCR 4) did not influence C4bp binding to either strain (gray shaded area) when compared with binding of pure C4bp alone (dashed lines).