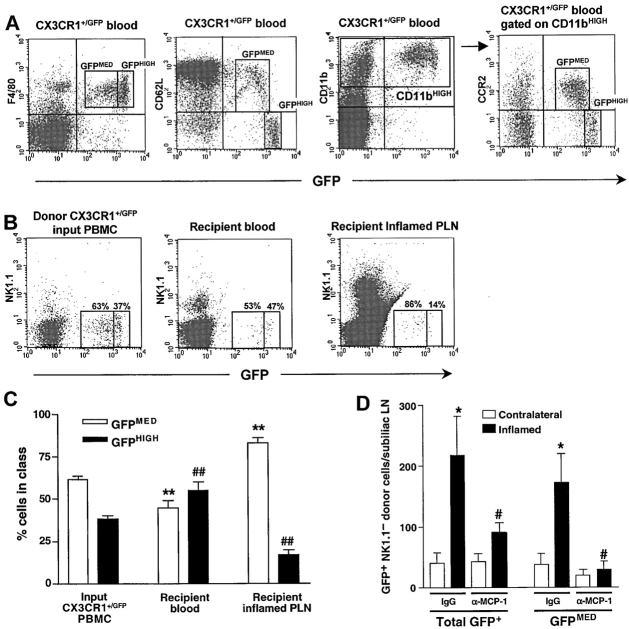
Figure 2.
Short-term homing of adoptively transferred L-selectin+CCR2+ monocytes to inflamed PLNs is mediated by MCP-1. (A) Flow cytometry of PBMCs from CX3CR1+/GFP knockin mice stained for the monocyte-macrophage markers F4/80 or CD11b (Mac-1) reveals two populations of GFP+ leukocytes based upon differential expression of GFP, L-selectin (CD62L), and CCR2. The F4/80+GFP− cells were identified as eosinophils by their high side scatter (data not shown). (B) Flow cytometric analysis of leukocyte populations in homing assays. CX3CR1+/GFP PBMCs were injected intravenously into wild-type recipients 5–7 d after induction of skin inflammation by CFA/KLH injection. 4 h later, GFP+ donor populations in recipient blood and inflamed PLNs were compared with input PBMCs. To avoid counting of contaminating GFP+ NK cells, all samples were stained with anti-NK1.1. Numbers in dot plots indicate the percentage of GFP+ cells in the GFPMED and GFPHIGH gates in one representative experiment (out of 6). (C) GFPMED cells (white bars), but not GFPHIGH cells (black bars) were preferentially recruited to inflamed PLNs. Data presented as mean ± SEM, n = 6 mice per group. **P < 0.01 vs. frequency of input GFPMED CX3CR1+/GFP PBMCs in the input. ## P < 0.01 vs. input frequency of GFPHIGH CX3CR1+/GFP PBMCs. (D) Homing of all GFP+ and GFPMED CX3CR1+/GFP NK1.1− leukocytes to inflamed subiliac PLNs (black bars) and to contralateral noninflamed PLNs (white bars). The inflammation-induced increased homing of the GFPMED subset was completely blocked by anti–MCP-1. Anti–MCP-1 treatment did not affect homing of GFPHIGH cells and did not alter CX3CR1+/GFP NK1.1− cell numbers in recipient blood or spleen (data not shown). Data presented as mean ± SEM, n = 5 mice per group. *P < 0.05 control mAb inflamed versus contralateral PLNs. # P < 0.05 vs. control mAb-treated inflamed PLNs.