Figure 3.
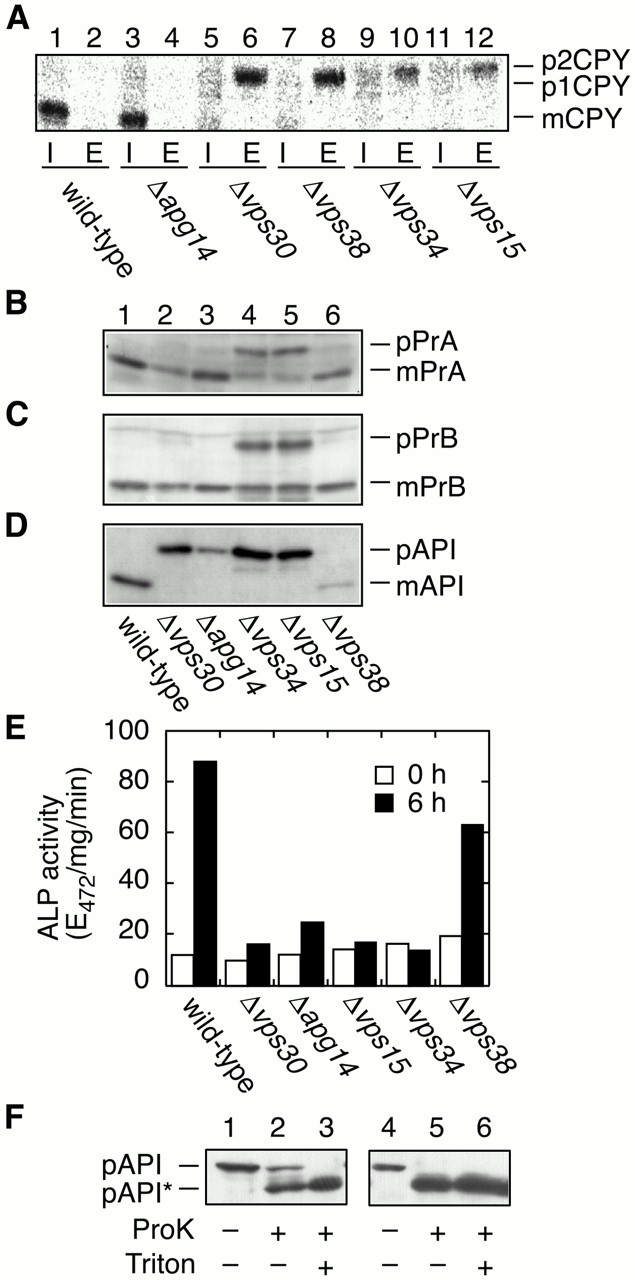
Transport of vacuolar proteins and autophagy. TN125 (wild type), AKY13 (Δapg14), AKY15 (Δvps30), AKY114 (Δvps38), AKY109 (Δvps34), and AKY115 (Δvps15) cells were grown in SC medium lacking methionine (A) or in YPD (B–D) at 28°C. (A) Yeast cells were labeled with [35S]methionine/cysteine for 15 min and chased with unlabeled methionine and cysteine for 30 min. The labeled cells were converted to spheroplasts and separated into pellet (I, intracellular) and supernatant (E, extracellular) fractions. CPY was immunoprecipitated and visualized by autoradiography using BAS2000. (B–D) Total protein was separated by SDS-PAGE and detected by immunoblotting with anti-PrA (B), anti-Pr B (C), and anti-API (D). (E) Cells were grown in YPD (open bars) and shifted to SD(-N) medium for 6 h (filled bars) at 28°C. Lysates from each group of cells were subjected to the ALP assay (Noda and Ohsumi 1998) to measure autophagy activity. (F) KVY4 (Δypt7; lanes 1–3) and AKY131 (Δvps34; lanes 4–6) cells grown in YPD to a log phase were transferred to SD(-N), and incubated for 4.5 h at 30°C. Total lysates were centrifuged at 13,000 g for 15 min. The pellets were treated with or without Triton X-100 and/or proteinase K as indicated on ice for 30 min. The samples were TCA-precipitated and subjected to immunoblotting with anti-API antibodies. pAPI*, digested pAPI fragment.
