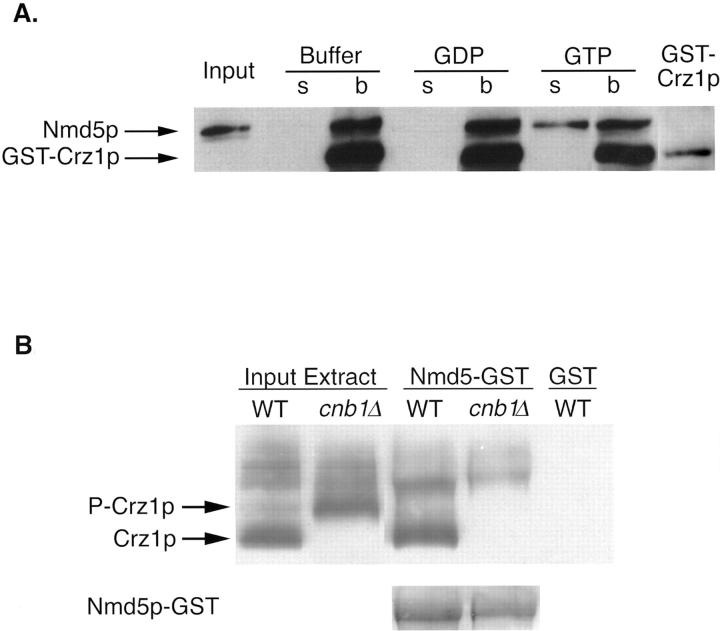
Figure 4.
Crz1p and Nmd5p form an import complex. (A) Nmd5p binding to Crz1p is disrupted by Gsp1p–GTP. 5 μg GST–Crz1p was bound to glutathione resin and incubated with 5 μg thrombin-cleaved Nmd5p. After extensive washing, the resin was incubated with 10 μM Gsp1p(Q71L)–GTP, Gsp1p(Q71L)–GDP, or buffer alone. The supernatant was collected, and the washed resin was resuspended in Laemmli loading buffer. Equivalent amounts of the bound and unbound fractions were analyzed on a 6% SDS-PAGE followed by Western blotting using an anti-Nmd5p antibody that also recognizes GST (GST–Crz1p is shown as an internal control). (B) Nmd5p binds Crz1p in a calcineurin-dependent manner. 200 μg yeast cytosol from crz1Δ strains ASY472 (WT) and ASY475 (cnb1Δ) expressing HA-tagged Crz1p (pAMS451) were incubated with 50 μl Nmd5p–GST bound to glutathione resin. Equal amounts of the bound fractions were analyzed by Western blotting using an anti-HA antibody. The mobility of HA-Crz1p was compared with untreated yeast cytosol (Input Extract). As a control, WT yeast cytosol was incubated with GST-bound resin. The bottom displays a Ponceau stain analysis of the Western blot to demonstrate equivalent amounts of Nmd5p–GST.