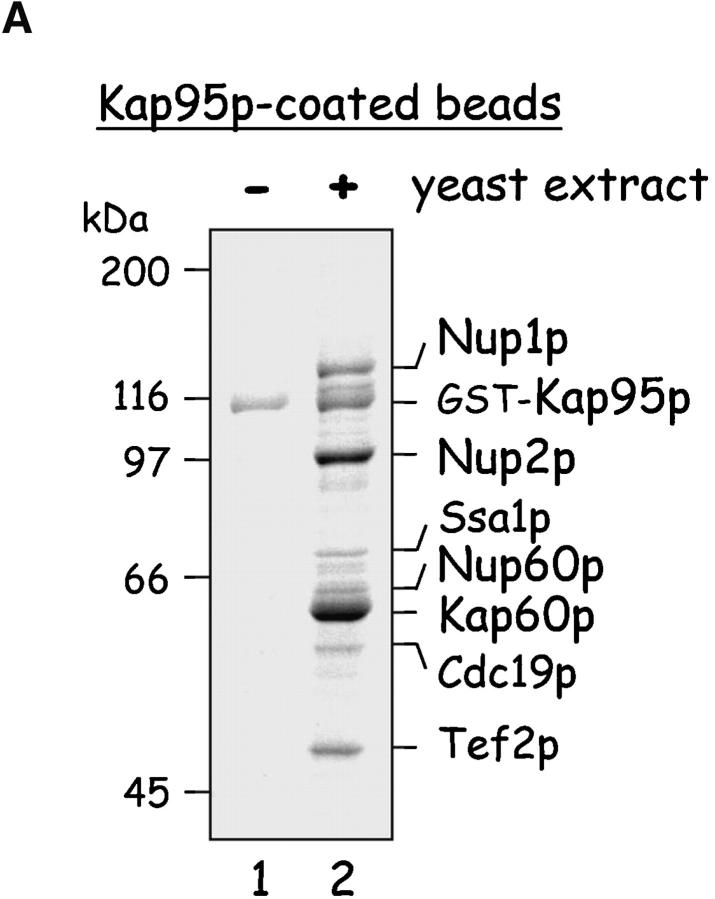
Figure 1.
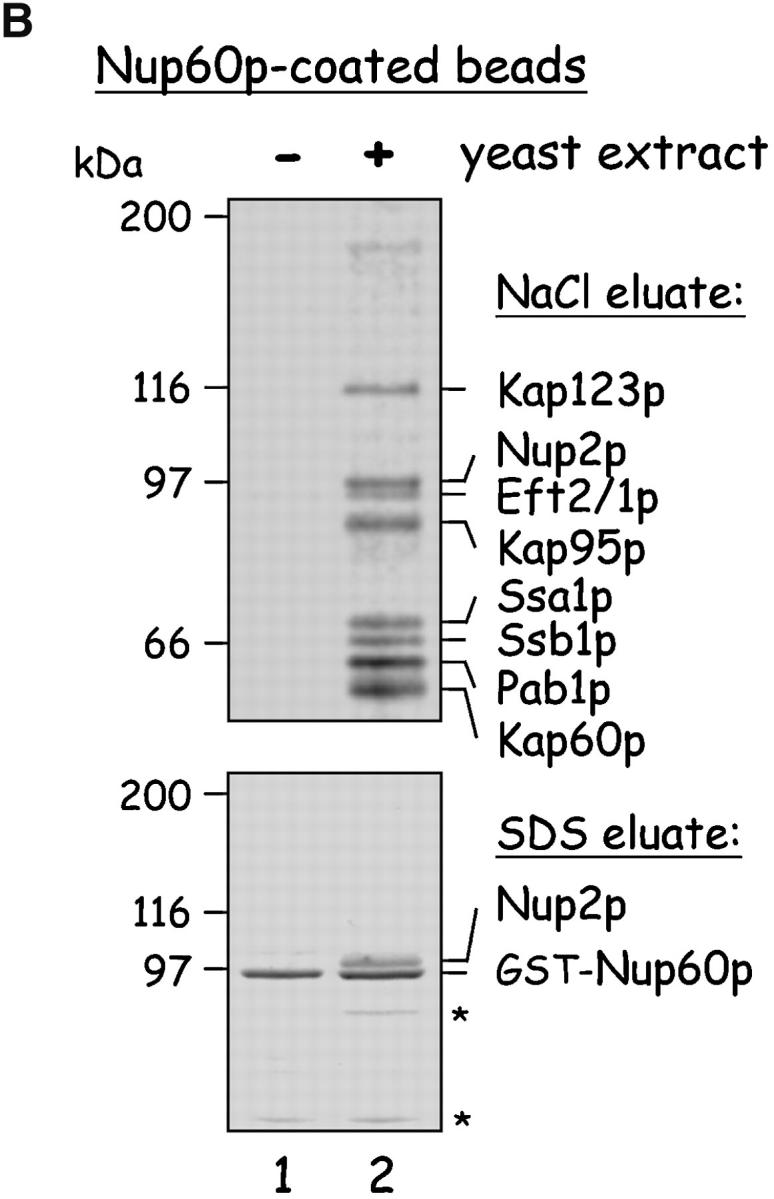
Yeast proteins that bind Nup60p and Kap95p. (A) Proteins in yeast extracts captured on Kap95p-coated Sepharose beads. GST-Kap95p (5 μg) was immobilized on glutathione-coated Sepharose beads (beads) and incubated with yeast extract (10 mg protein) or buffer as indicated. After washing beads, bound proteins were eluted with 250 mM MgCl2, collected by precipitation with trichloroacetic acid and deoxycholate, resolved by SDS-PAGE, and stained with Coomassie blue. Visible proteins were identified by mass spectrometry (see Materials and methods). Note that the three Nups captured by Kap95p are components of the nuclear basket structure of the yeast NPC. (B) Proteins in yeast extracts captured on Nup60p-coated beads. GST-Nup60p (1 μg) was immobilized on the beads and incubated with yeast extract (10 mg protein) or buffer as before. Bound proteins were eluted with 1 M NaCl (top) followed by SDS (bottom) and were identified as before. The asterisks mark a degradation product of GST–Nup60p. The identity of the ∼180-kD protein in the top panel could not be determined. Note that a portion of Nup2p remained tightly bound to Nup60p-coated beads even after incubation in 1 M NaCl.