Figure 3.
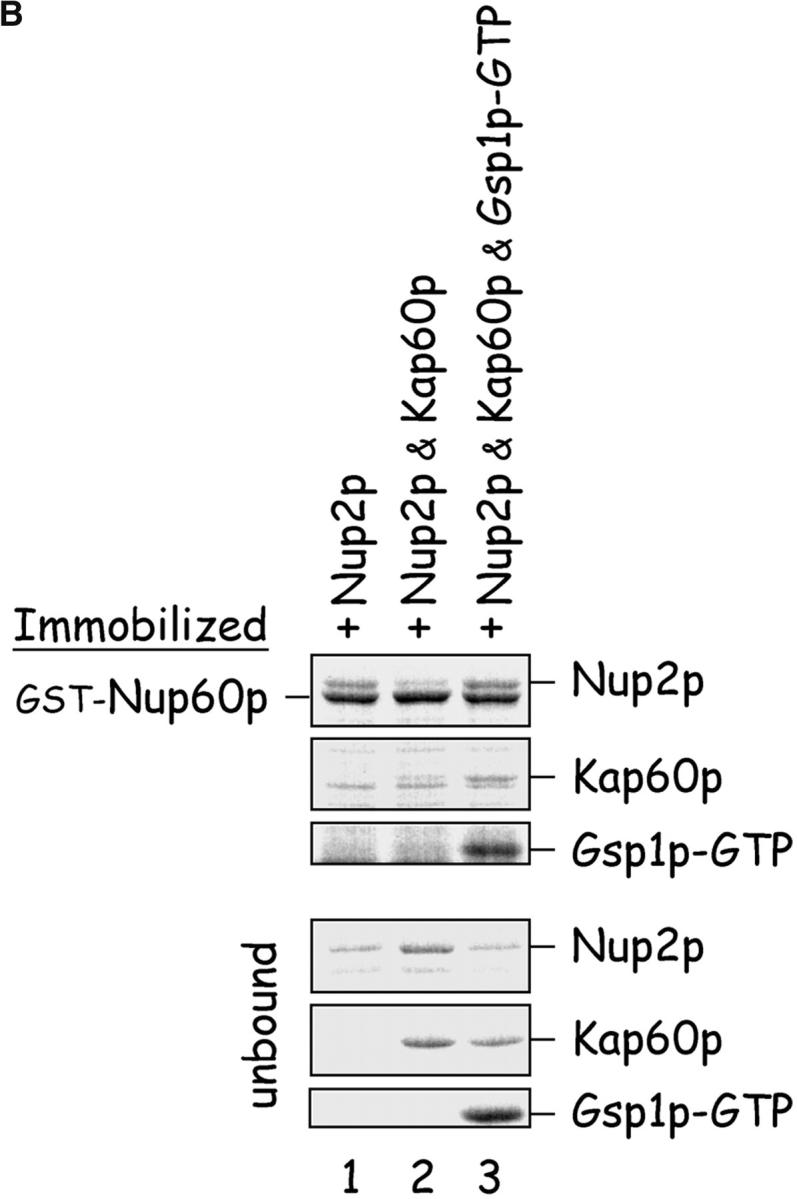
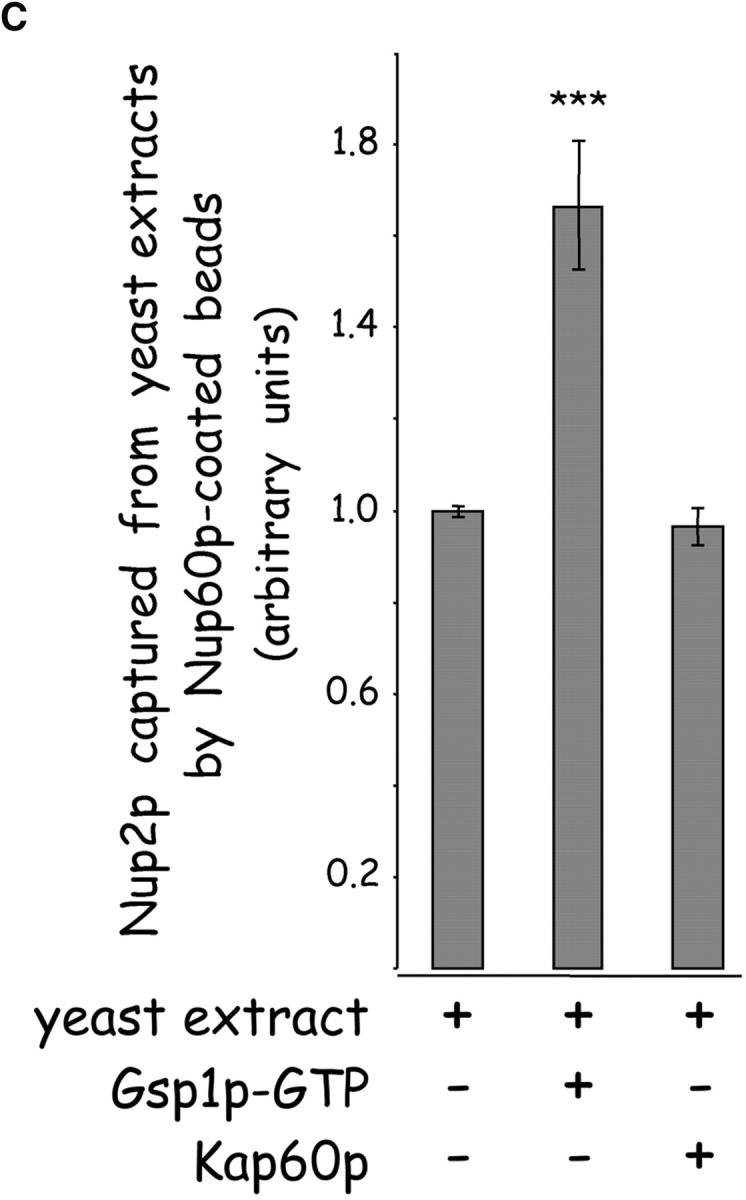
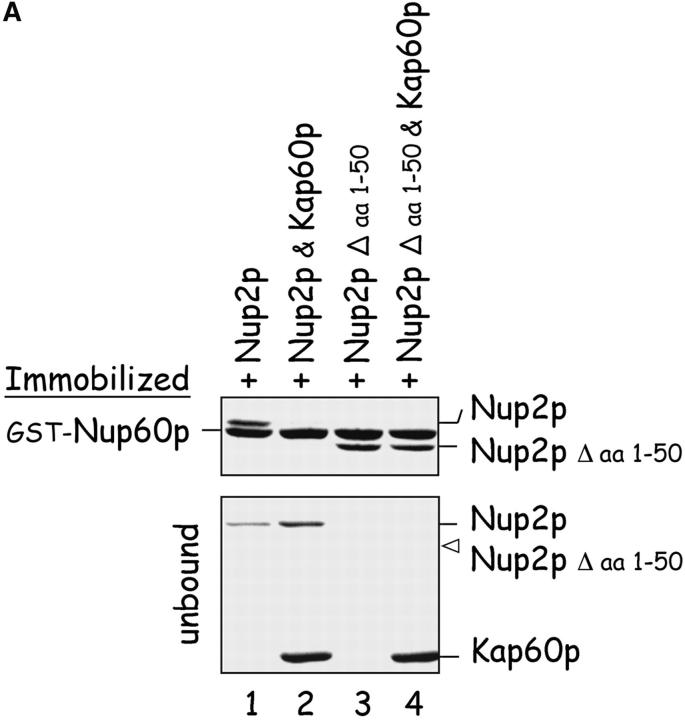
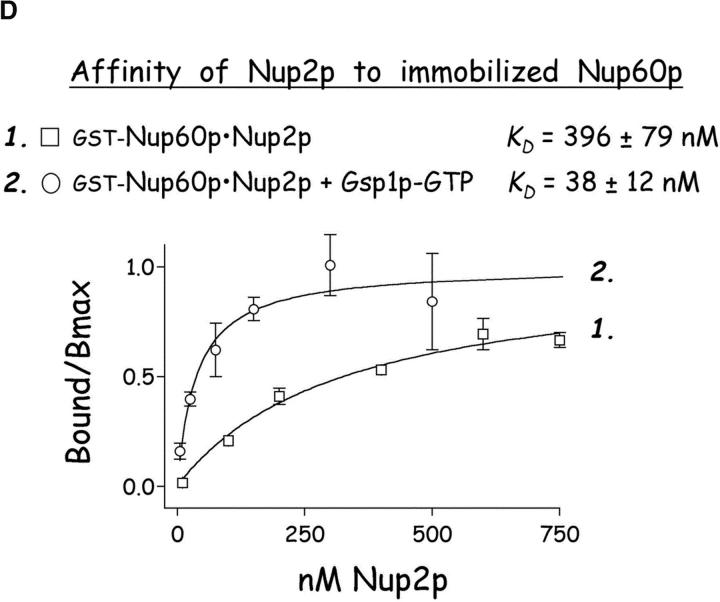
Gsp1p–GTP and Kap60p modulate the interaction between Nup60p and Nup2p. (A) The interaction between Nup2p and Nup60p, and the effect of Kap60p. GST–Nup60p (1 μg) was immobilized on beads and incubated with Nup2p (0.5 μg), Nup2pΔ (aa 1–50) (0.5 μg), or Kap60p (1 μg) as indicated. After 1 h at 4°C, unbound and bound proteins were collected, resolved by SDS-PAGE, and visualized with Coomassie blue. Note that Nup2p binds Nup60p, that Kap60p prevents the interaction, and that the NH2 terminus of Nup2p is not required for binding Nup60p. (B) Effect of Gsp1p–GTP on the interaction between Nup2p and Nup60p. GST–Nup60p (1 μg) was immobilized on beads and incubated with Nup2p (0.5 μg), Kap60p (1 μg), or Gsp1p–GTP (His-Gsp1p Q71L) (1 μg) as before. Note that Kap60p interferes with the interaction of Nup2p with Nup60p, but that the presence of Gsp1p–GTP restores binding and promotes formation of Nup60p–Gsp1p–Nup2p–Kap60p complexes. (C) Gsp1p–GTP enhances binding of Nup2p to Nup60p in yeast extracts. GST-Nup60p (1 μg) was immobilized on beads and was incubated with yeast extract (∼1 mg) supplemented with 1.25 μM recombinant Gsp1p–GTP (Q71L), 0.5 μM recombinant Kap60p, or no additional protein. The amount of Nup2p bound to Nup60p-coated beads was determined by quantitative Western blotting as described in Materials and methods. The amount of Nup2p was expressed as the ratio of Nup2p bound per unit of immobilized GST–Nup60p, using the incubation of extract without additions as baseline. Shown are the mean ratios for two samples with error bars representing the SEM; this experiment was performed three times with similar results. The asterisks (***) indicate a P < 0.05 for comparison of mean Nup2p captured from extracts supplemented or not with additional Gsp1p–GTP (unpaired, two-tailed t test). Note that addition of Gsp1p–GTP to yeast extract increases by ∼65% the amount of Nup2p bound to Nup60p-coated beads. (D) Gsp1p–GTP increases the affinity between Nup60p and Nup2p. Nup60p-coated beads were incubated with various concentrations of radiolabeled Nup2p for 2 h at 25°C in binding buffer with 10 mg/ml BSA and protease inhibitors. The concentration of GST–Nup60p within the beads was 25 nM and 150 nM for experiments with or without Gsp1p–GTP, respectively. The dissociation constant (KD) of the Nup60p–Nup2p complex in the presence and absence of 3 μM Gsp1p–GTP Q71L was calculated as described in Materials and methods. To facilitate comparison, the results were plotted as a fraction of maximal Nup2p bound versus Nup2p concentration. Each data point was performed in duplicate and error bars represent SEM. Note the 10-fold higher affinity between Nup60p and Nup2p in the presence of Gsp1p–GTP.