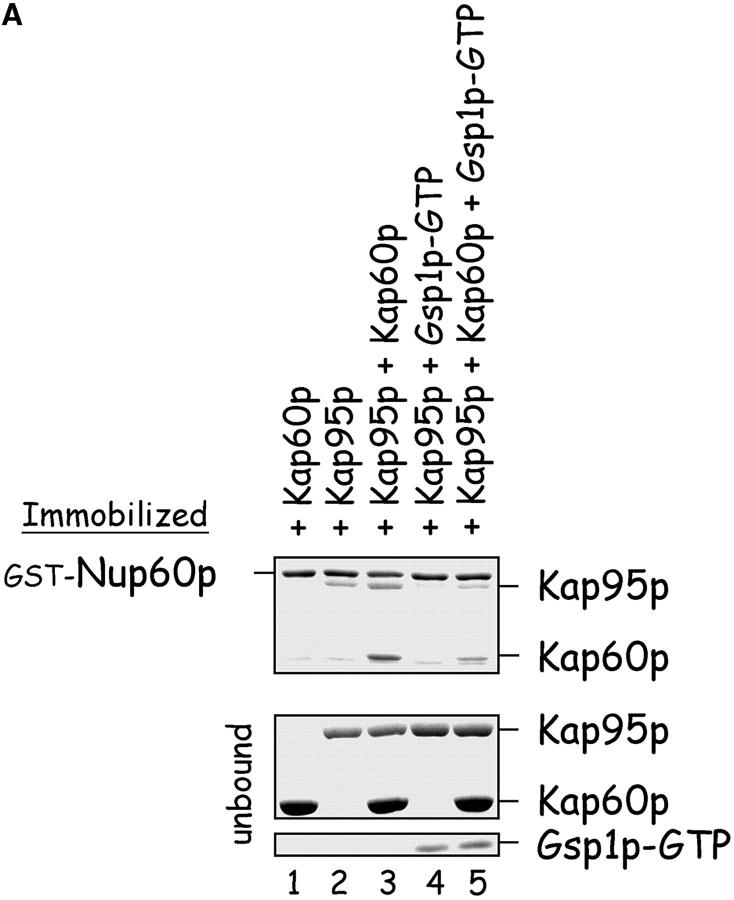
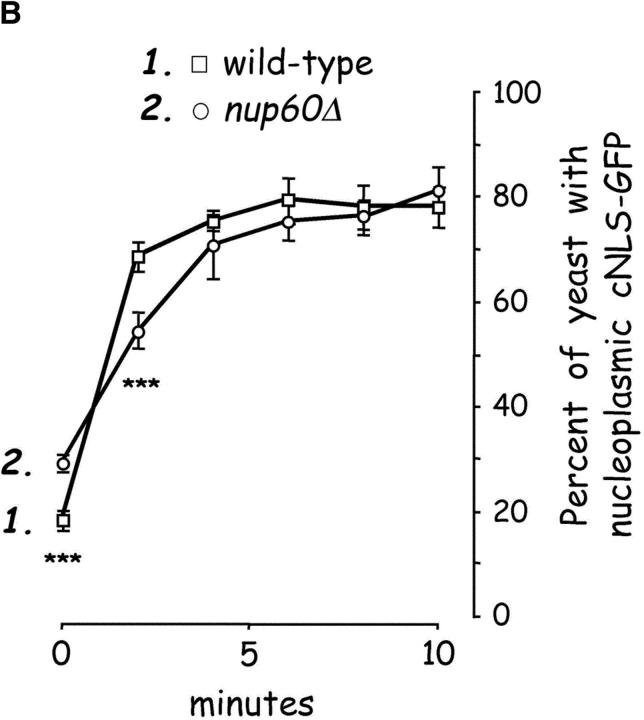
Figure 5.
Nup60p is a docking site for Kap95p–Kap60p heterodimers. (A) Nup60p binds Kap95p, but only in the absence of Gsp1p–GTP. GST–Nup60p (1 μg) was immobilized on beads and incubated with Kap95p (2 μg), Kap60p (3 μg), and/or Gsp1p–GTP Q71L (0.5 μg). After 1 h at 4°C, unbound and bound proteins were collected, resolved by SDS-PAGE, and stained with Coomassie blue. Note that Kap95p monomers bind to Nup60p, that Kap60p enhances binding of Kap95p to Nup60p, and that Gsp1p–GTP blocks binding of Kap95p and Kap95p–Kap60p heterodimers to Nup60p. (B) Nup60p plays a minor role in Kap95p–Kap60p-dependent import of cNLS-bearing cargo into the nucleus. Wild-type and nup60Δ yeast expressing the SV-40 T-antigen NLS fused to GFP were metabolically poisoned to deplete intracellular ATP and assayed for recovery of nuclear import upon removal of the poison (see Materials and methods). Values plotted at the indicated time points represent the mean fraction of yeast with predominantly nucleoplasmic NLS-GFP from six separate experiments (error bars represent SEM). The asterisks (***) indicate P < 0.01 for comparison of the two values at the indicated time points (unpaired, two-tailed t test). Note that yeast lacking Nup60p exhibit a slower initial rate of cNLS–GFP nuclear import.