Figure 7.
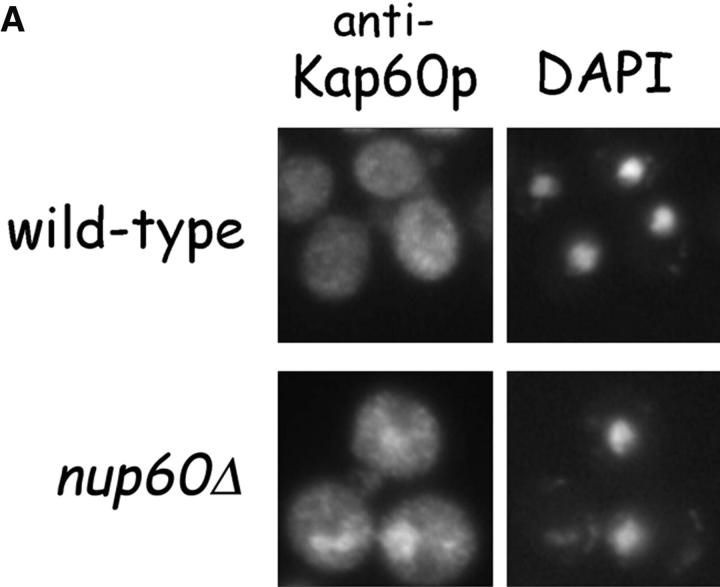
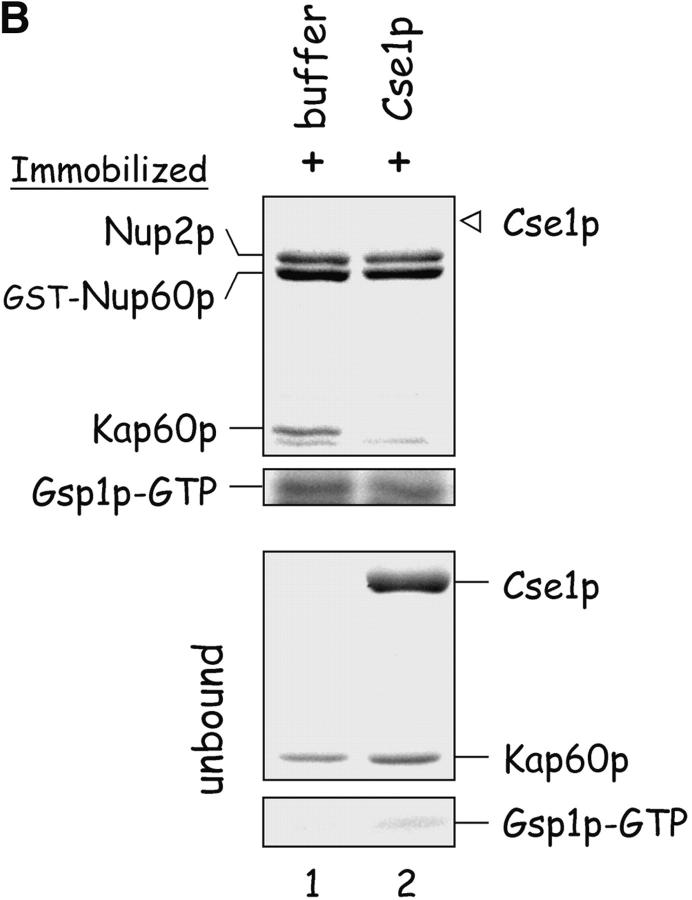
Nup60p plays a role in the nuclear export of Kap60p. (A) The location of Kap60p in wild-type and nup60Δ yeast was detected by indirect immunofluorescence using affinity-purified anti-Kap60p antibodies. Yeast grown to early log phase at 30°C in rich media were fixed in 3.7% formaldehyde for 1 h and processed for immunofluorescence microscopy (left). Note the moderate accumulation of Kap60p in nuclei of nup60Δ yeast compared with wild-type. (B) Cse1p accepts Kap60p and Gsp1p–GTP from a donor Nup2p–Gsp1p–GTP–Nup2p–Kap60p complex in vitro. GST–Nup60p (1 μg) was immobilized on beads and incubated with Nup2p (2 μg), Gsp1p–GTP (Q71L) (2 μg), and Kap60p (2 μg) for 1 h at 4°C to form the Nup2p–Gsp1p–GTP–Nup2p–Kap60p complex. After washing the beads to remove unbound proteins, the quaternary complex was mixed with buffer of Cse1p (1 μg). After 1 h at 4°C, unbound and bound proteins were collected, resolved by SDS-PAGE, and stained with Coomassie blue. Note that when Cse1p is present, all of the Kap60p and some Gsp1p are lost from the immobilized Nup60p. Also note that Cse1p does not bind to Nup60p–Nup2p complexes.