Figure 3.
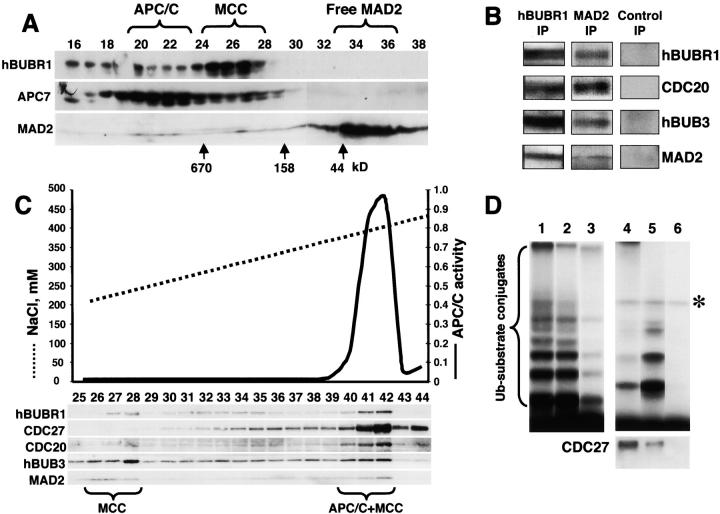
APC/C inhibitor is a complex of mitotic checkpoint proteins. (A) MAD2 comigrates with the hBUBR1 kinase complex. Mitotic HeLa extracts were separated through a Superose 6 column and the column fractions were probed for MAD2, hBUBR1, and the APC7. Fractions exhibiting APC/C inhibitory activity are denoted as MCC. (B) MCC consists of hBUBR1, hBUB3, MAD2, and CDC20. Fractions eluting from the 300–400-kD region of the Superose 6 column in A that exhibited APC/C inhibitory activity were pooled and immunoprecipitated with nonimmune IgG, anti-hBUBR1, and anti-MAD2 antibodies, washed, and probed with for hBUBR1, hBUB3, MAD2, and CDC20. (C) MCC can exist independently of APC/C. Fractions 19–28 from the Superose 6 column shown in A were combined and rechromatographed through a MonoQ anion exchange column by FPLC. Fractions were assayed for APC/C activity (solid line) and probed for hBUBR1, hBUB3, CDC20, MAD2, and CDC27 by Western blots. (D) Separation of active and inactive APC/C. Fractions 40–42 from the MonoQ column shown in C that exhibited APC/C activity and MCC were immunodepleted successively with anti-hBUBR1 and anti-CDC20 antibodies. The ubiquitin ligase activity was tested in the supernatants and immunoprecipitates. (Lane 1) Input APC/C activity; (lanes 2 and 3, respectively) APC/C activity in the supernatants after depletion of hBUBR1 and CDC20; (lanes 4 and 5, respectively) APC/C activity associated with corresponding hBUBR1 and CDC20 immunoprecipitates; and (lane 6) immunoprecipitate performed with nonimmune IgG. hBUBR1, CDC20, and nonimmune IgG immunoprecipitates were probed for CDC27 to estimate relative amount of APC/C (bottom panel). Asterisk denotes the contaminant iodinated protein.