Figure 5.
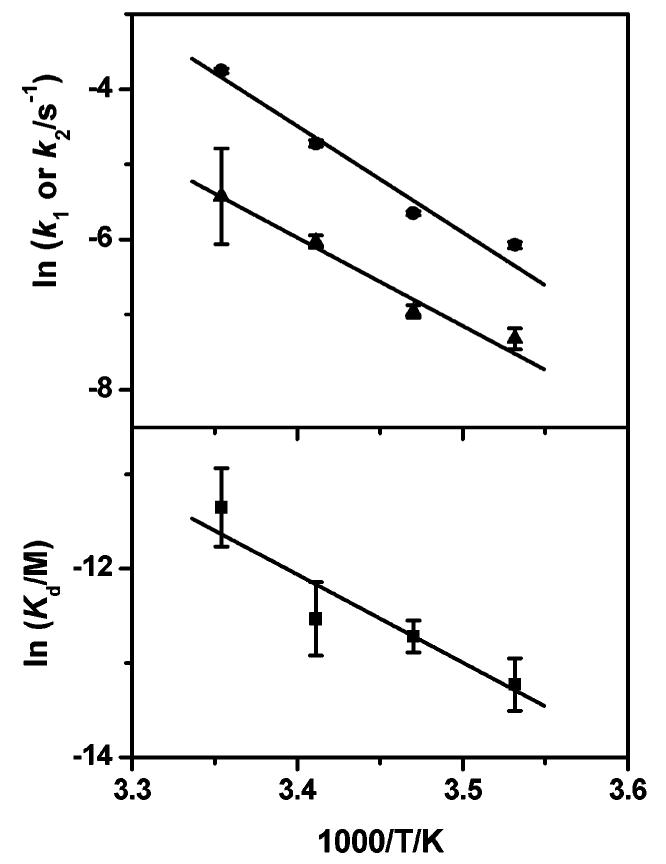
Top: Arrhenius plots of the opening rates of I:II (k1, ●) and HC·I:II (k2, ▲), respectively. Bottom: van't Hoff plot of the dissociation constant (Kd, ■) of HC to I:II. Activation energies for k1 and k2 obtained from the fits are 118 ± 2 and 98 ± 10 kJ mol−1, respectively, and the thermodynamic parameters found are ΔH = −77 ± 22 kJ mol−1 and ΔS = −163 ± 75 J mol−1 K−1.
