Figure 1.
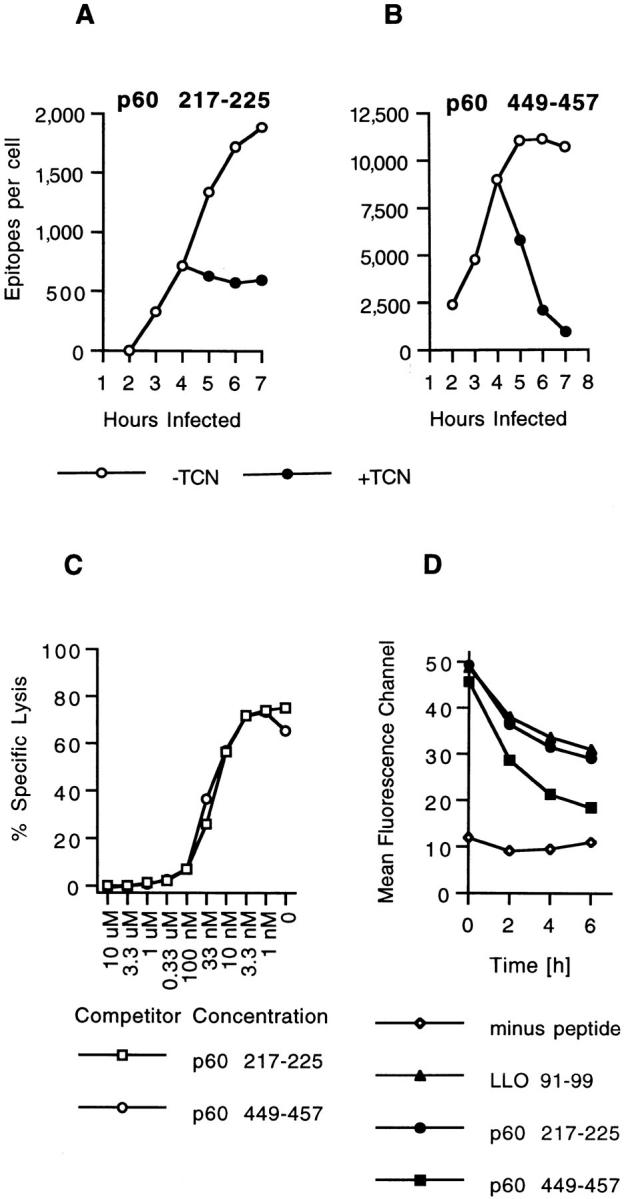
Two H2-Kd–binding p60 epitopes accumulate with different kinetics in infected cells. (A and B) J774 cells were infected with L. monocytogenes and p60 epitope accumulation followed in the absence and in the presence of 20 μg/ml tetracycline (TCN), a specific inhibitor of bacterial protein synthesis. TCN was added 4 h after infection. Cells were harvested at the indicated time periods, peptides were acid extracted, HPLC fractionated, and the amount of p60 217-225 and p60 449-457 was determined with CTL clone L9.6 (A) and WP11.12 (B). Epitope numbers were quantified and expressed as epitopes per infected cell. (C) Comparison of the binding affinities of p60 217-225 and p60 449-457 for H2-Kd molecules. P815 target cells were coated with 10−10 M LLO 91-99 in the presence of a range of p60 peptide concentrations and tested for recognition by LLO 91-99–specific CTL clone L12.3 as described in Materials and Methods. (D) Stability of H2-Kd-Listeria peptide complexes on the cell surface of RMA-S cells. Class I expression on RMA-S Kd cells was induced by incubation in the presence of 30 μM synthetic LLO 91-99, p60 217-225, or p60 449-457 at 37°C o/n. Control cells were incubated in the absence of peptide. Cells were chased at 37°C in the absence of peptide, and the stability of surface H2-Kd molecules was determined with H2-Kd–specific antibody SF1-1.1.1 and flow cytometry as described in Materials and Methods. Mean fluorecence channels of 10,000 gated events are depicted on the y-axis. Similar results were obtained in three independent experiments.
