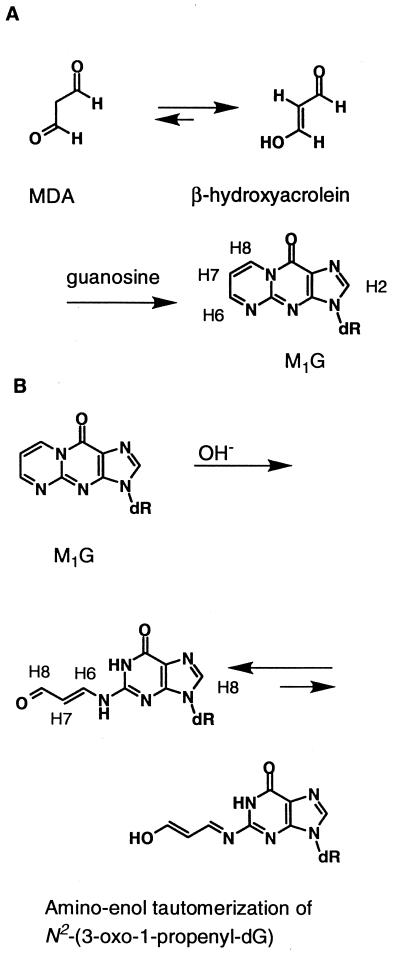
Figure 1.
(A) Formation of M1G from [1,2a] cyclization of deoxyguanosine by β-hydroxyacrolein. (B) Ring-opening to the N2-(3-oxo-1-propenyl)-dG derivative occurs at basic pH; the ring-opened species equilibrate between the favored amino and the enol tautomers. Note the numbering system for the M1G protons. In oligodeoxynucleotides, M1G is designated as M, and N2-(3-oxo-1-propenyl)-dG is designated as X.