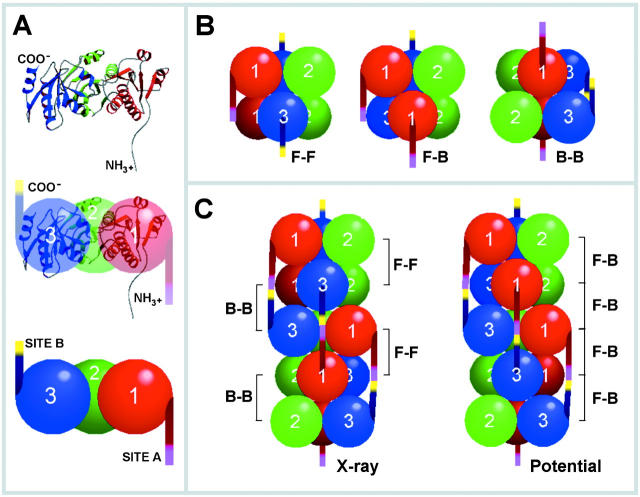
Figure 7.
Model for the CSQ oligomerization process. (A) MolScript (PDB1a8y) and sphere representation of the three domains of CSQ: red, domain 1 (residues 12–124); green, domain 2 (residues 125–228); blue, domain 3 (residues 229–352). The residues 326–333, which were not defined in the x-ray structure (Wang et al., 1998), are built manually using the Biopolymer option in InsightII software. (B) Representation of CSQ dimers. The NH2- and COOH-terminal tails are colored red and blue, respectively. The front-to-front dimer is shown at left, the back-to-back dimer at right, and the front-to-back dimer at center. (C) Regular repeating oligomers of CSQ. The oligomer shown at left is the front-to-front and back-to-back form revealed by x-ray structure (Wang et al., 1998). The oligomer shown at right is the front-to-back repeating form. In both oligomer forms, domain 2 has the same regular repeating spiral orientation. In contrast, the orientation of domains 1 and 3 differs in the two oligomeric forms. In the x-ray revealed oligomer, parallel spirals composed by alternating domain 1 followed by domain 3 are seen. In the potential oligomer, spirals of domain 1 and 3 run parallel to each other.