Figure 1. TIMP-2−/− myotubes are larger than wild-type myotubes when cultured in the presence of horse serum.
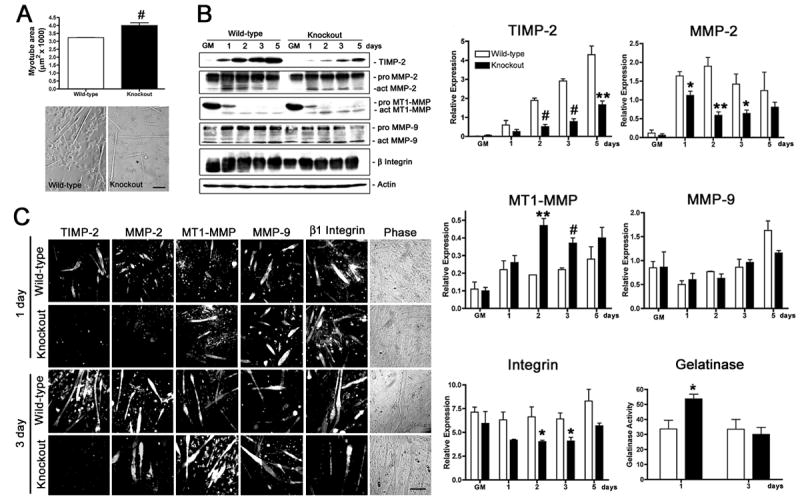
A) After 3 days in differentiation media containing 2% heat-inactivated horse serum, cells were fixed, and myotube area was measured (at least 20 randomly selected myotubes were measured from four wild-type and three TIMP-2−/− cultures). Data are presented as mean ± s.e.m. TIMP-2−/− myotubes are larger than wild-type myotubes. Myonuclei number is also increased in TIMP-2−/− myotubes, suggesting increased fusion (not shown). B) Western blot analysis with whole cell lysates (25 μg protein) of rapidly proliferating cells maintained in growth media (GM) for 24 hours after plating and after 1, 2, 3, and 5 days in differentiation media containing 2% heat-inactivated horse serum. Densitometric analysis (normalized to actin at each time point) reveals a dramatic reduction in TIMP-2 and active MMP-2 expression, increased active MT1-MMP expression, and no change in active MMP-9 expression in TIMP-2−/− myotubes. Similar to TIMP-2−/− muscle in vivo, total cellular β1 integrin expression, as well as cell surface expression (not shown), is decreased in TIMP-2−/− myotubes at 3 days, when myotube size is increased. Using a fluorescently caged gelatin substrate (i.e., DQ gelatin), protease activity was measured in 100 μl conditioned media (normalized to unconditioned medium containing serum). No difference in net gelatinolytic activity is detected at 3 days; in contrast, gelatinolytic activity is significantly increased at 1 day, the period of myoblast migration and fusion. Data are presented as mean ± s.e.m. C) Immunocytochemistry of Triton permeabilized cells confirms the western blot data of decreased TIMP-2 and MMP-2 expression and increased MT1-MMP expression in TIMP-2−/− myotubes. *p < 0.05, **p ≤ 0.01, #p ≤ 0.001. Scale bar = 100 μm.
