Figure 3.
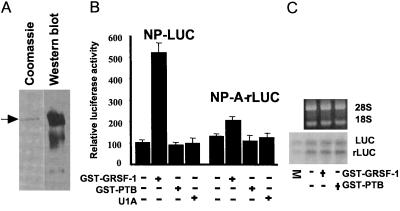
GRSF-1 selectively enhances translation of the influenza viral NP 5′ UTR-driven template. (A) GST-GRSF-1 fusion proteins (0.200 μg) were visualized by Coomassie staining or Western blotting. The arrow indicates the intact GST-GRSF-1 fusion protein. (B) The NP 5′ UTR-driven (NP-LUC) and mutant NP 5′ UTR-driven (NP-A-rLUC) templates (0.125 μg each) were translated in a HeLa extract in the absence or the presence of GST-GRSF-1 (0.200 μg). GST-PTB fusion protein (0.200 μg) and U1A (0.200 μg) again were utilized as specificity controls. After 45 min at 30°C, translation products were assayed by using a Dual-Luciferase Reporter Assay System (Promega). Values are the mean ± SD of three experiments per group. A scintillation counter was used to measure luciferase activity. Counts per minute (cpm) were produced by calculating the square root of measured cpm minus background cpm. We arbitrarily assigned a value of 100 to the control NP-luciferase reaction that, in this case, represented an average value of 3,560 cpm of luciferase activity per μl of HeLa extract. Other relative luciferase activity values were calculated relative to this number. (C) GST-GRSF-1 does not affect stability of template RNAs. Aliquots of translation products in B were extracted with phenol and phenol/chloroform, and RNAs were fractionated by formaldehyde-agarose gel electrophoresis. After electrophoresis, gels were stained with ethidium bromide to visualize ribosomal RNAs (18S and 28S, Upper) for internal controls. Template RNAs, which had been radiolabeled with trace amounts of 32P, then were visualized on x-ray film after gels were dried. LUC and rLUC indicate the firefly luciferase and the sea pansy luciferase RNA, respectively. M indicates mixture of an aliquot of the starting material of templates only.