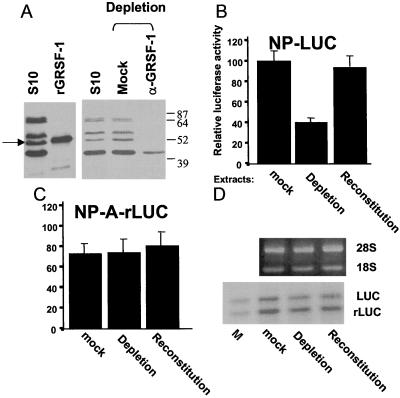
Figure 4.
Immunodepletion of GRSF-1 compromises translation of influenza virus 5′ UTR-driven chimeric mRNA translation, whereas GRSF-1 reconstitution restores mRNA translation. (A) HeLa S10 extracts (S10) were incubated with protein A-agarose beads coated with GRSF-1-specific IgG molecules (anti-GRSF-1) or with normal mouse IgG molecules (Mock) at 4°C for 4 hr. Depleted extracts then were centrifuged briefly, and the supernatant was examined by Western blotting. The GRSF-1 isoforms in the S10 starting material (100 μg) and the migration of the recombinant GRSF-1 (50 ng) are shown on the left. The GRSF-1 proteins patterned after depletion are shown on the right. (B and C) The mock-depleted, GRSF-1-depleted, or GRSF-1-reconstituted extracts were used for the cell-free translation of wild-type and mutant NP 5′ UTR-driven mRNA translation [NP-LUC (B) and NP-A-rLUC (C)]. For the GRSF-1-reconstituted extracts, 0.200 μg of GST-GRSF-1 fusion protein was added. After 45 min at 30°C, translation products were assayed by using a Dual-Luciferase Reporter Assay System (Promega). In this experiment, 100 was equivalent to an average of 2,340 cpm of luciferase activity per μl of HeLa extract. Mock, Depletion, or Reconstitution at the bottom indicates the mock-depleted, GRSF-1-depleted, or GRSF-1-reconstituted extract, respectively. Values are the mean ± SD of three experiments per group. (D) GST-GRSF-1 does not affect stability of template RNAs. Using aliquots (12 μl) of translation products (B and C), template RNA stability was tested as described in Fig. 3C. LUC and rLUC indicate the firefly luciferase and the sea pansy luciferase RNA, respectively. M indicates mixture of an aliquot of the starting-material templates.