Figure 1.
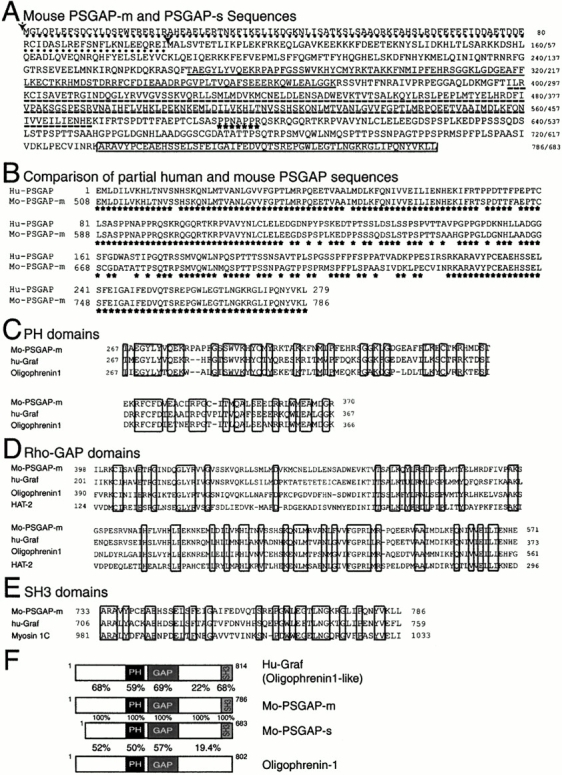
Cloning of PSGAP, a novel rhoGAP-containing protein that interacts with PYK2. (A) Deduced amino acid sequences of mouse PSGAP-m and PSGAP-s. Filled arrows indicate the two methionines as the translational starting codons for the splice variants, PSGAP-m (786 amino acids) and PSGAP-s (683 amino acids), respectively. PSGAP-m contains a different amino terminus (∼103 amino acids) that is underlined with dots. The PH domain (amino acids 267–370 in PSGAP-m) is underlined, rhoGAP domain (amino acids 398–571 in PSGAP-m) is underlined with dashed lines, the proline rich motif is indicated by stars, and the SH3 domain (amino acids 733–786 in PSGAP-m) is boxed. The original clone isolated by yeast two-hybrid screen contains amino acids 265–786. (B) Comparison of the partial human and mouse PSGAP protein sequences. Partial human PSGAP (hu-PSGAP) (279 amino acids) were obtained by searching human EST databases, which corresponded to the residues of 508–786 of mouse PSGAP-m (mo-PSGAP-m) with 84% identity. Stars indicate identical residues. (C) Sequence alignment of PH domains of mouse PSGAP-m, human Graf, and oligophrenin-1. (D) Sequence alignment of the RhoGAP domains of mo-PSGAP-m, hu-Graf, Oligophrenin-1, HAT-2. (E) Sequence alignment of the SH3 domains of mo-PSGAP-m, hu-Graf, and myosin 1C. Alignments were determined using the program pileup from the Genetics Computer Group. (C, D, and E) Identical residues are boxed. (F) Comparison of domain structures of mo-PSGAP-m, mo-PSGAP-s, hu-Graf, and oligophrenin-1. The PH, rhoGAP, and SH3 domains are indicated. The amino acid identities (%) within a specific domain are shown.
