Figure 4.
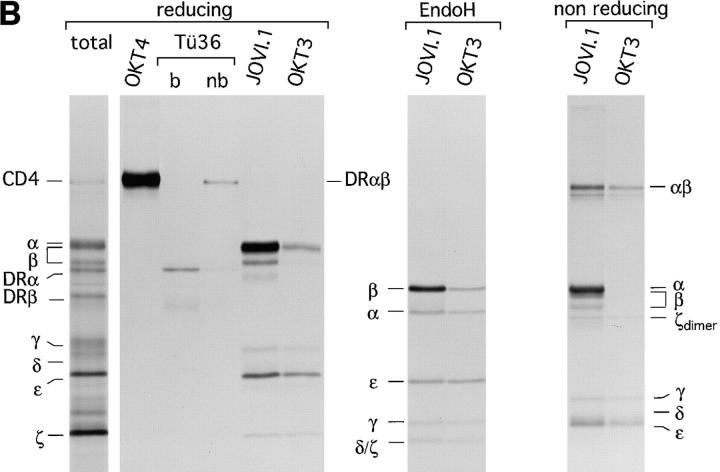
Assembly of the TCR–CD3 complex is a subunit-specific process and does not occur after lysis. (A) Test for antibody specificity confirms that in vitro assembly of the TCR–CD3 complex does not occur after lysis. (Left) Indicated mRNAs were translated individually for 1 h at 30°C, and an aliquot of the total microsome-associated material of each reaction was directly analyzed by reducing SDS-PAGE (12.5%). (Middle) Microsomes of individual translation reactions (left) were lysed in digitonin lysis buffer. Lysates were pooled to generate a mixture of labeled subunits, and subjected to immunoprecipitation using the antibodies indicated. (Right) Digitonin lysates of the individual translation reactions (left) were subjected to immunoprecipitation performed with a mixture of all antibodies used (middle), but lacking the indicated antibodies specific for the translated subunit. The lack of recovery of labeled subunits in this experiment indicates that none of the antibodies used react with the inappropriate subunits. (B) Subunit specific assembly of the TCR–CD3 complex in vitro. Nine mRNAs encoding all subunits of the TCR–CD3 complex as well as CD4 and HLA-DRα and HLA-DRβ were translated simultaneously. Microsomes were either analyzed directly by SDS-PAGE (12.5%) or subjected to immunoprecipitation using OKT4 (anti-CD4), Tü36 (anti–HLA-DR), JOVI.1 (anti–TCR-β) or OKT3 (anti-CD3). Precipitates obtained with OKT4 and Tü36 were analyzed by SDS-PAGE (12.5%) performed under reducing conditions. b, boiled; nb, non-boiled. SDS-stable DR dimers are indicated as DRαβ. Immunoprecipitates obtained with JOVI.1 and OKT3 were analyzed by SDS-PAGE (12.5%) performed under reducing and nonreducing conditions. To resolve TCR-α from TCR-β, these samples were also first digested with EndoH and then separated by SDS-PAGE (12.5%).